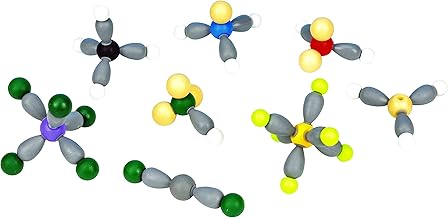
Electron domains are regions around a central atom where electrons are likely to be found. They are important in determining the electron domain geometry, which influences the molecular geometry of a molecule. Electron domain geometry considers both bonding and non-bonding electron domains to determine the geometrical shape. The molecular geometry, on the other hand, is the arrangement of atoms in a molecule, taking into account only the bonding electron domains. The valence-shell electron-pair repulsion (VSEPR) theory is used to predict the geometry of molecules based on their electron-domain geometry.
| Characteristics | Values |
|---|---|
| Definition | Number of lone pairs or bond locations around a particular atom in a molecule |
| Regions | Regions in a molecule where electrons are most likely to be found |
| Bonds | Single, double, triple |
| Lone Pairs | Two electrons not involved in bonding |
| VSEPR Theory | Used to predict molecular geometry |
Explore related products

Single bonds
A single bond is a type of chemical bond where two atoms share electrons with each other. Single bonds occur when one pair of electrons is shared between atoms as part of a molecule or compound. Single covalent bonds are sigma bonds, which occur when one pair of electrons is shared between atoms. Sigma bonds are the strongest type of covalent bond, formed by the direct overlap of orbitals from each of the two bonded atoms. Sigma bonds can occur between any kind of atomic orbitals. The only requirement is that the atomic orbital overlap happens directly between the nuclei of atoms.
Single covalent bonds can be represented by a single line between the two atoms. For example, the diatomic hydrogen molecule, H2, can be written as H—H to indicate the single covalent bond between the two hydrogen atoms.
French Constitutional Council: Term Limits and Tenure
You may want to see also

Double bonds
In the context of electron domains, double bonds are crucial for understanding molecular geometry. They are considered a single electron domain irrespective of having four bonding electrons. This is because they are localized to one region of space around the central atom. For example, in ethylene (C₂H₄), the C=C double bond counts as one electron domain, while each C-H bond counts as another, resulting in three electron domains for the carbon atom.
The Valence Shell Electron Pair Repulsion (VSEPR) theory suggests that electron domains repel each other and try to be as far apart as possible. Double bonds introduce a tweak in the shape of molecules as they have varying repulsive strengths compared to nonbonding electron domains. For instance, a molecule with four equally spaced electron domains will form a tetrahedron. However, the presence of a double bond or a nonbonding electron domain alters the shape due to their unique repulsive forces.
Furthermore, double bonds play a significant role in predicting molecular geometry. The convention is to use X to indicate the number of bonding electron pairs, E for the number of lone electron pairs, and A for the central atom of the molecule (AXnEm). For example, CO2 has two electron domains around the central carbon atom, with each double bond counting as one electron domain. This knowledge helps determine the expected geometry of a molecule, such as linear or bent, based on the arrangement of electron domains.
Woodrow Wilson's Stance on the US Constitution
You may want to see also

Triple bonds
In chemistry, an electron domain refers to the number of locations in the valence shell of an atom where electrons are likely to be found. Electron domains may also be referred to as electron groups. The number of electron domains indicates how many electrons surround a central atom. This, in turn, relates to the expected geometry of a molecule. Electron domains can be in the form of a single bond, a double bond, a triple bond, or a lone pair of electrons.
Many elements beyond oxygen can form triple bonds, and they are common in some transition metals. Hexa(tert-butoxy)ditungsten(III) and Hexa(tert-butoxy)dimolybdenum(III) are examples of compounds with metal-metal triple bonds.
Who's the Most Powerful Person in Congress?
You may want to see also
Explore related products

Nonbonded electrons
The concept of nonbonded electrons is crucial in understanding molecular geometry and the distribution of electrons within a molecule. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, nonbonded electrons contribute to the total count of electron domains. Electron domains refer to the regions in a molecule where electrons are most likely to be found, including single bonds, double bonds, and lone pairs. By considering the arrangement of electron domains, we can predict the geometry of a molecule.
In Lewis structures, nonbonding electrons are used to satisfy the octets of atoms within a molecule. The octet rule states that atoms tend to have eight electrons in their valence shell to be stable, similar to the noble gases. By distributing nonbonding electrons to achieve a full valence shell, molecules can attain a more stable configuration.
The presence of nonbonded electrons can vary depending on the molecule's structure and the number of covalent bonds present. For example, in a molecule with three covalent bonds, six valence electrons become bonding electrons, leaving six nonbonding electrons to satisfy the octets of the atoms.
Understanding FedEx's Additional Handling Fee Criteria
You may want to see also

Bonded electrons
In chemistry, electron domains refer to the regions in a molecule where electrons are most likely located. These domains can be in the form of single bonds, double bonds, triple bonds, or lone pairs of electrons. Single bonds between two atoms involve the sharing of two electrons, creating one electron domain. Double bonds consist of four shared electrons between two atoms, but they still count as one electron domain as they occupy a single region in space. A triple bond is also considered an electron domain.
Lone pairs refer to two electrons that are not involved in bonding. They count as one electron domain because they occupy space around the atom where electrons are present. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, electron domains arrange themselves as far apart as possible to minimize repulsion. This theory is used to determine the molecular geometry of a molecule. The convention is to indicate the number of bonding electron pairs by the letter X, the number of lone electron pairs by the letter E, and the letter A for the central atom of the molecule (AXnEm).
The number of electron domains indicates the number of places electrons are expected to be found around a central atom. This, in turn, relates to the expected geometry of a molecule. For example, in a water molecule (H₂O), the two bonding pairs (the O-H bonds) represent single bonds, while the two lone pairs on oxygen represent additional electron domains, totalling four electron domains. In ethylene (C₂H₄), the C=C double bond counts as one electron domain, while each C-H bond counts as another, giving a total of three electron domains for that carbon atom.
The sharing of electrons between atoms allows them to "stick" together and form molecules. This is the basis of covalent bonding. For instance, two chlorine atoms can share their unpaired electrons by forming a covalent bond and creating Cl2, allowing them to complete their valence shell. Each hydrogen atom in H2 shares a bonding pair with oxygen, resulting in a full valence shell of two electrons.
Constitution and Colonists: Did It Achieve Their Ideals?
You may want to see also
Frequently asked questions
An electron domain is a region around a central atom where electrons are likely to be found.
An electron domain consists of nonbonded electrons (lone pairs) and bonded electrons (covalent bonds).
No, an electron domain does not include the central atom itself.
Electron domains are crucial in determining the electron domain geometry, which influences the molecular geometry of a molecule. Electron-domain geometry considers both bonding and non-bonding electron domains to determine the geometrical shape.