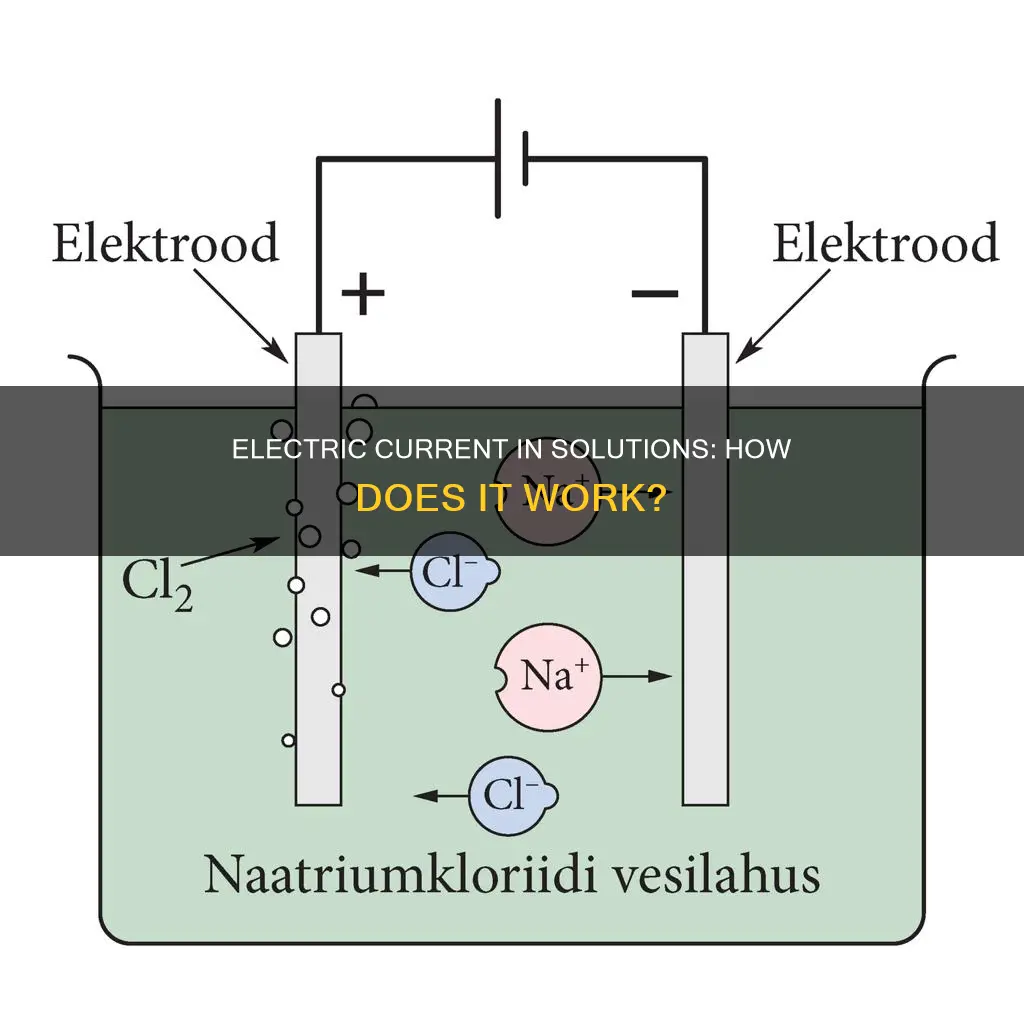
Electric current is the flow of charged particles, such as electrons or ions, through an electrical conductor or space. In an electric circuit, the charge carriers are often electrons moving through a wire. In semiconductors, they can be electrons or holes. In a solution, when an ionic compound dissolves, it dissociates into its constituent ions. These ions, which can be positively charged (cations) or negatively charged (anions), are then free to move within the solution. The movement of these ions within the solution creates an electric current. This occurs when a potential difference is applied across the solution using electrodes, causing the cations to be attracted to the negatively charged electrode (cathode) and the anions to be attracted to the positively charged electrode (anode).
| Characteristics | Values |
|---|---|
| Definition | A flow of charged particles |
| Charge Carriers | Electrons or ions |
| Direction of Current Flow | Opposite to the direction of electron flow |
| Ions | Positively charged (cations) or negatively charged (anions) |
| Electrodes | Cations are attracted to the negatively charged electrode (cathode) |
| Anions are attracted to the positively charged electrode (anode) | |
| Solutions in Contact | Required for the flow of electric current between two solutions |
| Half-Reaction at Cathode | Involves the reduction of ions or molecules in the solution |
| Electrolytes | Charge carriers are ions; in plasma, they are ions and electrons |
| Unit of Measurement | Ampere (amp) |
Explore related products
What You'll Learn

Ions and their movement
An electric current in a solution is constituted by the movement of ions within the solution. When an ionic compound dissolves, it dissociates into its constituent ions, which can be positively charged (cations) or negatively charged (anions). These ions are then free to move within the solution.
The movement of ions in a solution is facilitated by the presence of an electric field created by a potential difference applied across electrodes. The cations are attracted to the negatively charged electrode (the cathode), while the anions are drawn to the positively charged electrode (the anode). This movement of ions creates an electric current in the solution.
For an electric current to flow between two different solutions, the solutions must come into direct contact. When the two solutions are in contact, ions from one solution can migrate to the other. This allows for the completion of the circuit and the flow of electric current between the two solutions.
The type of ions present in a solution influences its ability to conduct electricity. Solutions containing ions, such as sodium and chlorine ions, facilitate a more seamless flow of electric current compared to solutions with covalent bonds. For example, when sodium chloride dissolves in water, the sodium and chlorine atoms separate and become positively and negatively charged ions. This separation of charges enables the solution to conduct electricity effectively.
In certain contexts, the movement of ions gives rise to visible manifestations of electric current. In electrolyte mixtures, for instance, brightly coloured ions in motion make the current visible due to their slow progress.
George Washington: A Constitution Framer?
You may want to see also

Electrodes and their charges
Electrodes are essential components of electrochemical cells, and they play a crucial role in the flow of electric current in solutions. Each electrochemical cell has two electrodes: a positively charged cathode and a negatively charged anode. The names of these electrodes are dependent on the circumstances and the type of cell. In a vacuum tube or a semiconductor with polarity, the anode is the positive electrode, and the cathode is the negative electrode.
The electrons naturally flow from the negatively charged anode towards the positively charged cathode. This movement of electrons constitutes an electric current. The process of losing electrons, known as oxidation, occurs at the anode, while the process of gaining electrons, called reduction, takes place at the cathode. During the discharge of a battery, the positive electrode is the cathode, and the negative electrode is the anode. However, during the charge cycle, their roles are switched: the positive electrode becomes the anode, and the negative electrode becomes the cathode.
The performance of an electrode is determined by its internal structure and the distribution of its components. To ensure optimal conductivity, the conductive agent should be evenly distributed over the active material. Additionally, the electrode should adhere to the current collectors to prevent it from dissolving into the electrolyte. The density of the active material is also crucial, and a balance should be maintained between the amount of active material, the conductive agent, and the binder.
In some cases, electrodes are chemically modified to alter their physical, chemical, electrochemical, optical, electrical, and transportive properties. These modified electrodes are used for advanced research and investigation purposes. Lithium electrodes, for example, have been studied for the development of Li-ion batteries, which are widely used in mobile phones and electric cars. Researchers are continuously working to enhance the safety, efficiency, and cost-effectiveness of these batteries, with a particular focus on improving the performance of their electrodes.
Enumerated Powers: An Example and Its Significance
You may want to see also

Electric fields
An electric field, also called an E-field, is a physical field that surrounds electrically charged particles, such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) describes their capacity to exert attractive or repulsive forces on another charged object. Charged particles exert attractive forces on each other when their charges are opposite, with one being positive and the other negative, and repel each other when the charges are the same. These forces are mutual and require two charges to be present. Coulomb's law states that the greater the magnitude of the charges, the greater the force, and the greater the distance between them, the weaker the force. Therefore, the greater the charge of an object, the stronger its electric field.
The electric field is stronger near charged objects and weaker further away. They originate from electric charges and time-varying electric currents. Electric fields and magnetic fields are both manifestations of the electromagnetic field, and electromagnetism is one of the four fundamental interactions of nature. Electric fields are important in many areas of physics and are exploited in electrical technology. For example, in atomic physics and chemistry, the interaction in the electric field between the atomic nucleus and electrons is the force that holds these particles together in atoms.
Maxwell's laws confirm the principle of locality imposed by the special theory of relativity, which requires cause and effect to be time-like separated events where the causal efficacy does not travel faster than the speed of light. The general solutions of fields are given in terms of retarded time, indicating that electromagnetic disturbances travel at the speed of light. Advanced time solutions, which also provide a solution for Maxwell's law, are ignored as they are considered unphysical.
The magnitude of the overall E-field is the addition of the two E-fields caused by the charges. For example, two charges (q1 and q2) are located on the x-axis of a coordinate system, both positive, but with q2 having twice the magnitude of q1. The superposition of the fields shows an overall E-field along the –x axis.
Who Qualifies to be in the Presidential Cabinet?
You may want to see also
Explore related products
$34.87

Electrochemical cells
In a full electrochemical cell, one half-cell undergoes oxidation, losing electrons to its electrode, while the other half-cell undergoes reduction, gaining electrons from its electrode. This transfer of electrons creates a difference in charge between the two half-cells, establishing an electric current.
One example of an electrochemical cell is a fuel cell, which uses hydrogen fuel and oxygen to generate electricity. Unlike batteries, fuel cells require a continuous supply of fuel and oxygen to sustain the chemical reaction and produce electricity continuously. Fuel cells are used in various applications, including backup power for buildings and powering vehicles, such as automobiles and submarines.
Another example is the electrolysis of water, where an electric current is passed through water to decompose it into hydrogen and oxygen. This process is used in electroplating, where a layer of metal, such as copper or silver, is deposited onto a surface using an electrolytic cell.
Blue Heeler-Aussie Mix: The Perfect Puppy?
You may want to see also

Conductivity
An electric current in a solution is constituted by the movement of electrically charged particles or ions within the solution. When an ionic compound dissolves, it breaks down into its constituent ions. These ions can be positively charged (cations) or negatively charged (anions). These ions are then free to move within the solution.
The movement of these ions creates an electric current in the solution. This movement occurs when a potential difference is applied across the solution using electrodes. The cations are attracted to the negatively charged electrode (the cathode), while the anions are attracted to the positively charged electrode (the anode). This migration of ions from one solution to another is facilitated by the electric field created by the potential difference applied across the electrodes.
The flow of ions and the transfer of charges can only occur when there is a pathway for the ions to move between the solutions. Therefore, for an electric current to flow between two different solutions, the solutions must come into actual contact with each other.
The conductivity of a solution depends on its composition. Solutions containing ions, such as sodium and chlorine ions, conduct electricity more effectively. When sodium chloride dissolves in water, the sodium and chlorine atoms separate and become positively and negatively charged ions. These ions can then move freely within the water, allowing the solution to conduct electricity.
On the other hand, substances with covalent bonds, such as sugar, do not conduct electricity well. When sugar dissolves in water, it does not break apart into ions. Instead, the electrons are shared among the atoms within the molecule, and no positive or negative charges are acquired. Therefore, solutions containing covalent compounds have higher resistance and are not good conductors of electric current.
Key Attributes of Effective Economic Theories and Models
You may want to see also
Frequently asked questions
An electric current in a solution is constituted by the movement of electrically charged particles, specifically ions, within the solution.
Ions are atoms that carry a positive or negative charge. They are formed when an ionic compound dissolves and breaks down into its constituent parts.
When a potential difference is applied across the solution using electrodes, the positively charged ions (cations) are attracted to the negatively charged electrode (cathode), while the negatively charged ions (anions) are attracted to the positively charged electrode (anode). This movement of ions creates an electric current in the solution.
Solutions that contain ions, such as sodium and chlorine, conduct electricity well. When dissolved in water, these ions are free to move around and carry a charge, allowing the solution to conduct electricity.











































