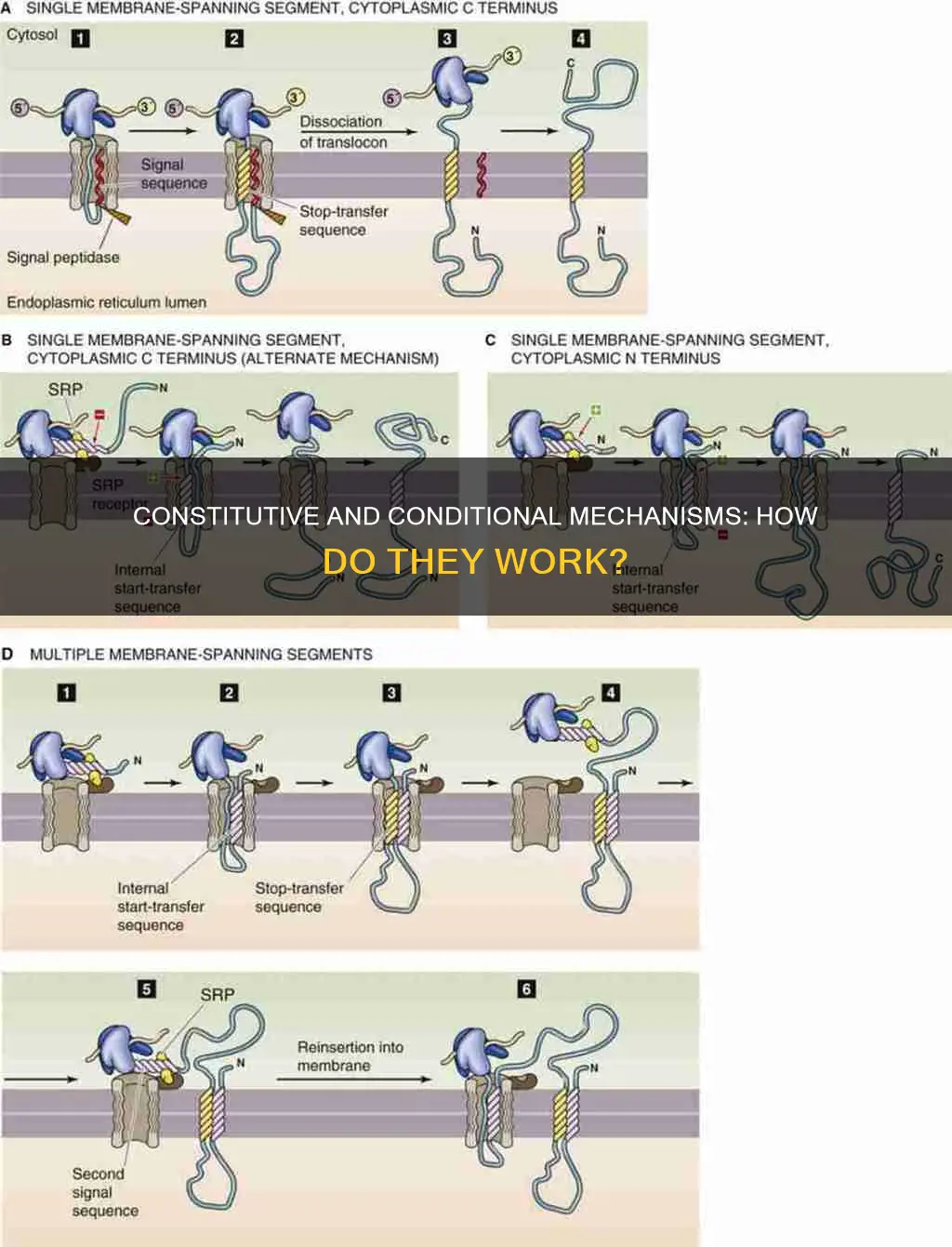
In cellular biology, active transport is the process of transporting molecules or ions across a cell membrane, from a region of lower concentration to a region of higher concentration, against the concentration gradient. Active transport requires cellular energy to achieve this movement and is essential for various physiological processes, such as nutrient uptake, hormone secretion, and nerve impulse transmission. There are two types of active transport: primary and secondary. Primary active transport uses adenosine triphosphate (ATP) to directly transport molecules and establish specific concentration gradients, while secondary active transport uses those established gradients to transport other molecules. Cotransporters, a category of transporters that includes symporters and antiporters, play a crucial role in this process. Meanwhile, conditional and constitutive mechanisms refer to the specificity and timing of gene inactivation, with conditional knockout allowing for more precise and targeted gene inactivation compared to constitutive knockout, which inactivates the gene in all cells and tissues.
Explore related products
What You'll Learn
- Passive transport: molecules move from high to low concentration without energy use
- Active transport: energy-driven process, moving molecules against the concentration gradient
- Cotransport: symporters and antiporters move molecules in the same or opposite directions
- ABC transporters: a protein family that imports/exports molecules across a cell membrane
- Gene inactivation: conditional knockout is more targeted and advantageous than constitutive knockout

Passive transport: molecules move from high to low concentration without energy use
Passive transport is the movement of molecules or ions across a cell membrane from a region of higher concentration to a region of lower concentration, without the use of cellular energy. This is in contrast to active transport, which requires energy to move molecules against the concentration gradient, from an area of low concentration to an area of high concentration.
Passive transport mechanisms do not require energy to function, unlike active transport mechanisms, which use adenine triphosphate (ATP) or an electrochemical gradient as a source of energy. Passive transport includes simple diffusion, where molecules such as gases (carbon dioxide and oxygen) and small molecules like ethanol, cross the cell membrane without assistance. This is a spontaneous process that continues until the concentration difference between the two regions is eliminated.
The rate of molecule transport across the membrane in passive transport can be influenced by the concentration gradient. When the concentration gradient is low, increasing it will lead to a higher rate of transport. This is because the movement of molecules is driven by the difference in concentration on either side of the membrane.
Osmosis is another example of passive transport, where water molecules pass through a membrane from a region of higher water concentration to a region of lower water concentration. Endosmosis is the process by which water molecules enter a cell, while exosmosis is the process by which they exit. Osmosis is influenced by factors such as gravity, mechanical pressure, and capillary action, and it plays a crucial role in maintaining water balance in cells.
Passive transport is essential for the exchange of materials into and out of cells. It allows necessary substances to enter the cell and waste products to exit, ensuring the cell's survival and proper functioning. This process is vital for cellular communication and maintaining the balance of dissolved materials within the cell.
Understanding Undue Preference Under Federal Power Act
You may want to see also

Active transport: energy-driven process, moving molecules against the concentration gradient
Active transport is an energy-driven process that moves molecules and ions across a cell membrane against the concentration gradient, from a region of lower concentration to a region of higher concentration. This process requires cellular energy, in contrast to passive transport, which allows molecules to move with the concentration gradient without the use of energy. Active transport is essential for various physiological processes, such as nutrient uptake, hormone secretion, and nerve impulse transmission.
There are two types of active transport: primary active transport and secondary active transport. Primary active transport uses adenosine triphosphate (ATP) to directly power the transport of molecules and establish specific concentration gradients. Examples include sodium/potassium-ATPase and hydrogen-ATPase pumps. ABC transporters, a diverse protein family, often function as ATP-driven pumps and are involved in the import or export of molecules across cell membranes.
Secondary active transport, on the other hand, employs established electrochemical gradients to transport molecules. It utilizes cotransporters, such as symporters and antiporters, to move multiple solutes in the same or opposite directions, respectively. An example of a symporter is the glucose symporter SGLT1, which co-transports one glucose molecule and two sodium ions in the same direction, from low to high concentration. In contrast, the antiporter mechanism allows the movement of one solute species from high to low concentration to drive the transport of another solute from low to high concentration. The sodium-calcium exchanger, for instance, transports three sodium ions into the cell to transport one calcium ion out.
The discovery of active transport mechanisms has significant implications for various fields. In 1926, Dennis Robert Hoagland's research on plants' ability to absorb salts against a concentration gradient contributed to our understanding of nutrient absorption and its dependence on metabolic energy. Later, Robert K. Crane's discovery of sodium-glucose cotransport as the mechanism for intestinal glucose absorption in 1960 marked the first proposal of flux coupling in biology. More recently, Jens Christian Skou received the Nobel Prize in Chemistry in 1997 for his research on the sodium-potassium pump, further highlighting the importance of active transport in cellular processes.
The Elastic Clause: A Flexible Constitution?
You may want to see also

Cotransport: symporters and antiporters move molecules in the same or opposite directions
Cotransporters are membrane proteins that transport multiple solutes across a cell membrane simultaneously. They can be classified as symporters or antiporters, depending on whether the substances they transport move in the same or opposite directions.
Symporters use the downhill movement of one solute species from high to low concentration to move another molecule uphill from low concentration to high concentration. Both molecules are transported in the same direction. An example is the glucose symporter SGLT1, which co-transports one glucose (or galactose) molecule into the cell for every two sodium ions it imports into the cell. This symporter is located in the small intestines, heart, brain, and kidneys. Its mechanism is exploited in glucose rehydration therapy, which uses the absorption of sugar through the walls of the intestine to pull water in along with it.
Antiporters, on the other hand, pump two species of ions or other solutes in opposite directions across a membrane. One of these species is allowed to flow from high to low concentration, which yields the entropic energy to drive the transport of the other solute from a low-concentration region to a high-concentration region. An example is the sodium-calcium exchanger, which allows three sodium ions to enter the cell in exchange for pumping one calcium ion out. This mechanism is important within the membranes of cardiac muscle cells to maintain low calcium concentration in the cytoplasm.
Cotransport is a form of active transport, which is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration, against the concentration gradient. Active transport requires cellular energy to achieve this movement and is essential for various physiological processes, such as nutrient uptake, hormone secretion, and nerve impulse transmission. It is primarily powered by adenosine triphosphate (ATP) hydrolysis, which establishes specific concentration gradients.
The Constitution and Racial Profiling: What's the Law?
You may want to see also
Explore related products
$18.95

ABC transporters: a protein family that imports/exports molecules across a cell membrane
ABC transporters, or ATP-binding cassette transporters, are a superfamily of integral membrane proteins that transport various substrates across cellular membranes. They are one of the largest and most ancient families of proteins, with representatives found in all extant phyla from prokaryotes to humans.
ABC transporters utilize the energy of adenosine triphosphate (ATP) binding and hydrolysis to carry out biological processes, including the translocation of substrates across membranes. The ABC transporter protein structure consists of at least four domains: two ABC or nucleotide-binding domains and two transmembrane domains (TMDs). The conserved ABC domains provide the nucleotide-dependent engine that drives transport, while the more variable TMDs create the translocation pathway.
ABC transporters can function as importers or exporters. In prokaryotes, importers mediate the uptake of nutrients, ions, amino acids, peptides, sugars, and other hydrophilic molecules into the cell. The membrane-spanning region of the ABC transporter protects these hydrophilic substrates from the lipids of the membrane bilayer, enabling their passage across the cell membrane. Eukaryotes do not possess any importers.
Exporters or effluxers are present in both prokaryotes and eukaryotes, where they function as pumps that extrude toxins, drugs, lipids, sterols, and metabolites out of the cell. In gram-negative bacteria, exporters also transport lipids and polysaccharides from the cytoplasm to the periplasm. Some human exporters are involved in tumor resistance, cystic fibrosis, and other inherited diseases.
A third subgroup of ABC proteins does not function as transporters but is involved in translation and DNA repair processes. These proteins are essential for cell viability, virulence, and pathogenicity. For example, bacterial iron ABC uptake systems are important effectors of virulence, as they scavenge iron using siderophores, which are high-affinity iron-chelating molecules.
Class Action Lawsuits: Who Can Be Included?
You may want to see also

Gene inactivation: conditional knockout is more targeted and advantageous than constitutive knockout
Gene knockouts, or gene inactivation, is a widely used genetic engineering technique that involves the targeted removal or inactivation of a specific gene within an organism's genome. There are two main types of gene knockouts: complete and conditional. While a complete gene knockout permanently inactivates the gene, a conditional gene knockout is more targeted and advantageous as it allows for the gene to be turned off and on at specific times or in specific tissues.
The main advantage of conditional gene knockout is its specificity. It allows researchers to study the role of individual genes in living organisms at specific stages of development. This is particularly useful when studying genes that may be essential for embryonic development but play a different role in adults. By using conditional gene knockout, scientists can avoid embryonic death from gene mutations, which is a limitation of traditional gene knockout methods.
Conditional gene knockout also eliminates many of the side effects associated with traditional gene knockout. In traditional gene knockout, the gene is inactivated in all cells and tissues, which can modify the animal's physiology, adaptation, and compensation mechanisms, resulting in false results. With conditional gene knockout, the gene can be selectively inactivated in specific tissues, allowing for a more precise understanding of gene function.
Additionally, conditional gene knockout provides flexibility in terms of timing. It allows researchers to knock out genes at specific times during development, enabling the study of gene function at different stages. This flexibility is particularly useful when investigating genes that may have varying roles at different stages of an organism's life cycle.
Overall, conditional knockout is a powerful tool in genetic research. Its specificity, ability to bypass limitations associated with constitutive knockout, and flexibility in timing make it a more targeted and advantageous approach compared to constitutive knockout. By using conditional knockout, researchers can gain deeper insights into gene function, particularly in the context of specific tissues and developmental stages.
Executive Powers: Constitutional Limits and Scope
You may want to see also