The Lac repressor is a DNA-binding protein that inhibits the expression of genes coding for proteins involved in the metabolism of lactose in bacteria. The Lac operon is an operon required for the transport and metabolism of lactose in E. coli and many other enteric bacteria. The Lac repressor-mediated regulation of transcription in the Lac operon is based on the high-affinity binding of the repressor protein to the Lac operator (O1) and the consequent steric hindrance to E. coli RNA Pol at the transcription initiation region. The Lac repressor is bound simultaneously to both the main operator O1 and either O2 or O3. The Lac operon is negatively controlled by an inducible system. This means that the Lac repressor is induced by the presence of an inducer molecule, such as allolactose, which causes an allosteric change in its shape, reducing its affinity for the operator and allowing transcription to occur.
| Characteristics | Values |
|---|---|
| Lac Repressor | LacI |
| Description | A DNA-binding protein that inhibits the expression of genes coding for proteins involved in the metabolism of lactose in bacteria |
| Gene Expression | The gene is mostly off in the absence of an inducer and mostly on in the presence of an inducer |
| Inducer | Allolactose or Isopropyl β-D-1-thiogalactopyranoside (IPTG) |
| Role | Ensures that the bacterium only invests energy in the production of machinery necessary for uptake and utilization of lactose when lactose is present |
| Binding Site | 22 base pairs (bp) long |
| Monomer | 360 amino acids |
| Atomic Mass | 154,520 Dalton |
| Search | 10-100 times faster than the theoretical upper limit for two particles searching for each other via diffusion in 3D |
Explore related products
What You'll Learn

Lac repressor's role in lactose metabolism
The lac repressor (LacI) is a DNA-binding protein that plays a crucial role in the metabolism of lactose in bacteria. It inhibits the expression of genes coding for proteins involved in lactose metabolism. This inhibition occurs when lactose is not available to the cell, ensuring that the bacterium only produces the machinery necessary for lactose uptake and utilization when lactose is present.
When lactose becomes available, it is converted into allolactose by β-Galactosidase (lacZ) in bacteria. Allolactose acts as an inducer, binding to the lac repressor and causing an allosteric change in its shape. This conformational change reduces the lac repressor's ability to bind tightly to its cognate operator, which is a specific DNA sequence known as the operator region of the lac operon. As a result, the genes coding for proteins involved in lactose uptake and utilization can be expressed.
The lac repressor operates through a helix-turn-helix motif in its DNA-binding domain. It binds specifically to the major groove of the operator region of the lac operon, with additional base contacts made by residues of symmetry-related alpha helices, known as "hinge" helices, which bind deeply in the minor groove. This binding mechanism ensures tight regulation of lactose metabolism.
The lac repressor also exhibits a unique search mechanism to locate its target operator DNA. In vitro studies have shown that this search process is significantly faster than the theoretical limit for two particles diffusing in three dimensions. It is hypothesized that LacI and other transcription factors find their binding sites through facilitated diffusion, a combination of free diffusion in 3D and 1D-sliding on the DNA helix. This sliding motion allows the repressor to track the major groove, extending the target length and enhancing the search process.
In summary, the lac repressor plays a critical role in lactose metabolism by regulating the expression of genes involved in lactose uptake and utilization. Its ability to bind DNA and respond to the presence of lactose ensures efficient metabolism and energy conservation in bacteria.
Individual Responses to Cues: Their True Nature Revealed
You may want to see also

Lac operon induction and repression
The Lac operon is a regulatory mechanism that allows bacteria to metabolise lactose when glucose is not available. It was first discovered by François Jacob and Jacques Monod, who won the Nobel Prize in Physiology in 1965 for their work. The Lac operon is a well-known example of prokaryotic gene regulation.
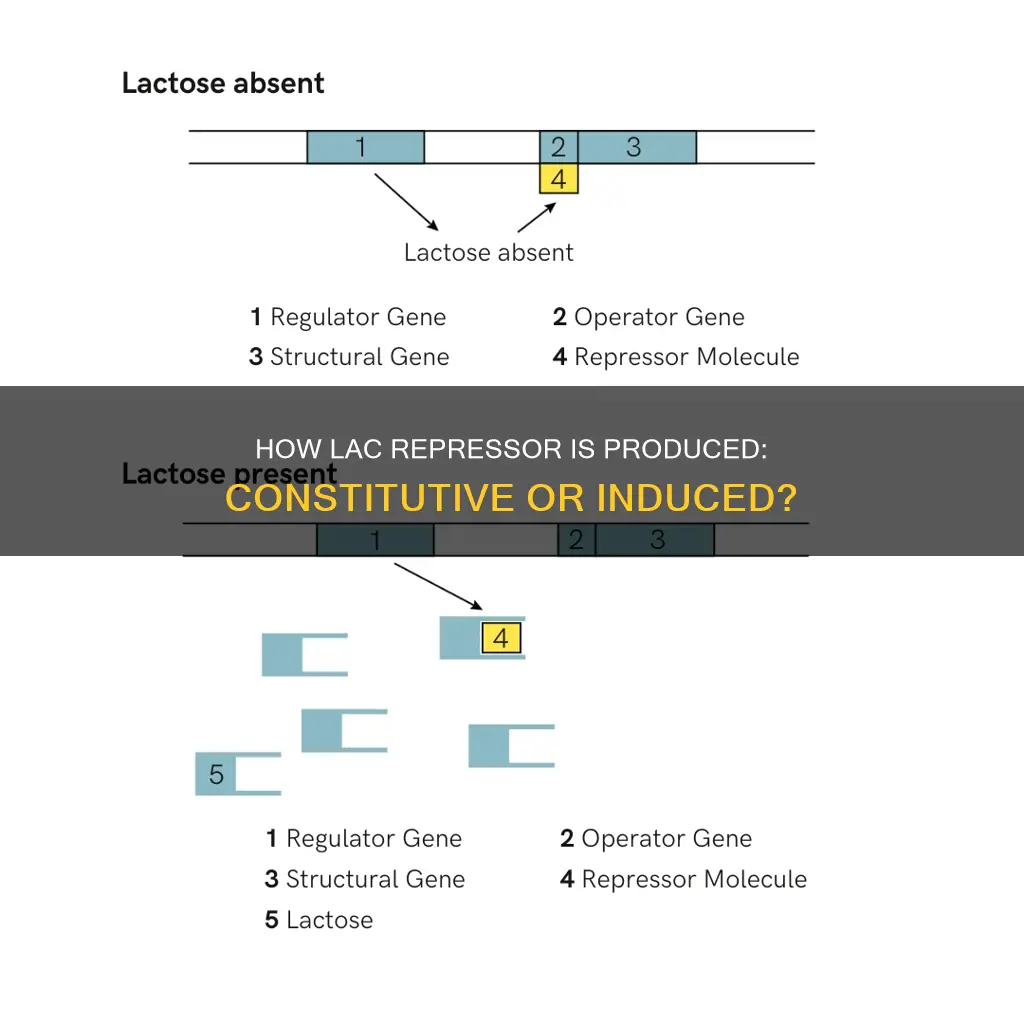
The Lac operon is regulated by the Lac repressor (LacI), a DNA-binding protein that inhibits the expression of genes coding for proteins involved in the metabolism of lactose in bacteria. LacI operates by binding to the operator region of the lac operon, preventing the expression of genes that code for proteins involved in lactose uptake and utilisation. This ensures that the bacterium only invests energy in the production of machinery necessary for lactose uptake and utilisation when lactose is present.
When lactose is present, it is converted into allolactose by β-galactosidase (lacZ) in bacteria. Allolactose binds to the Lac repressor, causing an allosteric change in its shape. This altered shape has a reduced affinity for the operator region of the lac operon, allowing the expression of genes involved in lactose metabolism. The presence of allolactose, therefore, acts as an inducer of the lac operon, promoting the expression of the genes necessary for lactose metabolism.
The induction of the lac operon can also be achieved artificially using isopropyl β-D-1-thiogalactopyranoside (IPTG), a commonly used allolactose mimic. IPTG can bind to the Lac repressor and induce a conformational change, reducing its affinity for the operator region. This allows for the transcription of genes being regulated by the lac repressor, even in the absence of lactose.
In summary, the Lac operon is a regulatory mechanism that allows bacteria to metabolise lactose efficiently. The Lac repressor plays a crucial role in this process by inhibiting the expression of lactose metabolism genes in the absence of lactose. When lactose is present, the Lac repressor undergoes a conformational change, allowing the expression of these genes. This induction process can also be artificially achieved using IPTG, providing a useful tool for studying gene regulation in bacteria.
Japan's Constitution: Limiting Government Power?
You may want to see also

Lac operon's role in glucose transport
The lactose operon (Lac operon) is a regulatory mechanism that allows for the transport and metabolism of lactose in E. coli and other enteric bacteria. The Lac operon is required for the effective digestion of lactose when glucose is not available.
The Lac operon consists of three structural genes: lacZ, lacY, and lacA. LacZ encodes β-galactosidase, an enzyme that cleaves lactose into glucose and galactose. LacY encodes β-galactoside permease, a membrane protein that facilitates the transport of lactose into the cell. LacA encodes β-galactoside transacetylase.
The Lac operon is regulated by the lac repressor (LacI), a DNA-binding protein that inhibits the expression of genes coding for proteins involved in lactose metabolism. LacI operates by binding to the operator region of the Lac operon, blocking the binding of RNA polymerase and preventing the transcription of Lac proteins.
In the absence of lactose, the Lac repressor halts the production of Lac enzymes and transport proteins. However, a minimal amount of gene expression still occurs, allowing the Lac operon to detect the presence of lactose. When lactose is available, it is converted into allolactose, which binds to the Lac repressor and causes a conformational change. This reduces the repressor's affinity for the operator region, allowing the expression of genes involved in lactose uptake and utilization.
In the presence of glucose, the Lac operon is repressed, regardless of the presence of lactose. This is due to the inactivation of the catabolite activator protein (CAP) and the shutdown of lactose permease by EIIAGlc, which prevents the transport of lactose into the cell. Thus, the Lac operon plays a crucial role in the transport and metabolism of lactose, particularly when glucose is unavailable, by ensuring the efficient utilization of lactose as an alternative carbon source.
The Constitution's Legacy: Years After Its Creation
You may want to see also
Explore related products
$104.1 $109.99

Lac repressor's structure and function
The lac repressor (LacI) is a DNA-binding protein that inhibits the expression of genes coding for proteins involved in the metabolism of lactose in bacteria. LacI operates by a helix-turn-helix motif in its DNA-binding domain, binding base-specifically to the major groove of the operator region of the lac operon. The lac operon was the first gene regulatory system to be discovered.
The structure of the Lac repressor was determined by X-ray crystallography and shows that it is a homotetrameric protein. Each monomer of 360 amino acid residues consists of five parts: a three-helix bundle "headpiece", a "hinge helix" that constitutes the DNA-binding domain (DBD), a core domain divided into N- and C-subdomains with an inducer-binding pocket at their junction, a linker that connects the DNA-binding domain with the core domain, and a C-terminal tetramerization region. The tetramer contains two DNA-binding subunits composed of two monomers each (a dimer of dimers).
The lac repressor plays a crucial role in gene regulation. When lactose is not available to the cell, the lac repressor binds to DNA and blocks the production of proteins that are encoded in the local area. This ensures that the bacterium only invests energy in the production of machinery necessary for lactose uptake and utilization when lactose is present. When lactose is available, it is converted into allolactose, which inhibits the DNA-binding ability of the lac repressor due to allosteric regulation. This allows genes coding for proteins involved in lactose uptake and utilization to be expressed.
The in vivo search model for the lac repressor includes intersegment transfer and hopping, as well as crowding by other proteins that make the genome in E. coli cells less accessible for the repressor. The lac repressor has been observed to bypass operators, flip orientation, and rotate with a longer pitch than the DNA while moving along it.
The Constitution: How Many Constituents Are Enough?
You may want to see also

Lac operon mutations and their effects
The lactose operon (lac operon) is required for the transport and metabolism of lactose in E. coli and other enteric bacteria. The lac operon and its regulators were first characterised by studying mutants of E. coli that exhibited abnormalities in lactose metabolism.
Some mutants expressed the lac operon genes constitutively, meaning the operon was expressed regardless of the presence of lactose. These mutants are called constitutive mutants. One example is Oc, in which a mutation in an operator sequence reduces or prevents the repressor (the lacI gene product) from recognising and binding to the operator sequence. Thus, in Oc mutants, lacZ, lacY, and lacA are expressed whether or not lactose is present.
Another type of mutant allele of lacI, called I-, prevents the production of a repressor polypeptide or produces a polypeptide that cannot bind to the operator sequence. This is also a constitutive expresser of the lac operon because the absence of repressor binding permits transcription.
The regulation of the lac operon can be further understood by using two copies of the operon sequences in one cell, resulting in a partial diploid in E. coli. This can be accomplished by using the F-factor to carry one copy, while the other is on the genomic E. coli chromosome. Researchers have used this genetic tool to create partial diploids (merozygotes) that allow them to test the regulation with different combinations of mutations in one cell. For example, the F-factor copy may have an IS mutation, while the genomic copy has an OC mutation. This can be used to determine that IS is dominant to I+, which is dominant to I-. It also shows that the OC mutation acts in cis, while the lacI mutation can act in trans.
In the presence of glucose, inducer exclusion blocks expression of the lac operon. Glucose transport into the cell drains the phosphate group from the PTS proteins, and the unphosphorylated form of EIIAGlc binds to the lac permease and prevents it from bringing lactose into the cell. Thus, if both glucose and lactose are present, the transport of glucose blocks the transport of the inducer of the lac operon.
Wisconsin vs. Yoder: Religious Freedom in the Constitution
You may want to see also
Frequently asked questions
The lac repressor (LacI) is a DNA-binding protein that inhibits the expression of genes coding for proteins involved in the metabolism of lactose in bacteria.
The lac repressor works by binding to the operator region of the lac operon, preventing the synthesis of lac mRNA and the production of the enzymes needed for lactose metabolism. When lactose is present, it is converted into allolactose, which binds to the lac repressor and changes its shape. This altered shape can no longer bind tightly to the operator, allowing the expression of genes involved in lactose uptake and utilisation.
Inducers, such as allolactose or IPTG, play a crucial role in the lac repressor system. They bind to the repressor molecule and cause a conformational change, reducing its affinity for the operator DNA. This allows transcription of the genes necessary for lactose metabolism to occur, ensuring that the bacterium only invests energy in lactose utilisation when lactose is available.

































