MHC class II molecules are a type of major histocompatibility complex (MHC) molecule that plays a crucial role in the immune system. These molecules are typically found on professional antigen-presenting cells (APCs), such as dendritic cells, macrophages, and B cells. The expression of MHC class II is tightly regulated, and its activation is essential for initiating immune responses by presenting antigens to T cells. Macrophages, a type of APC, express MHC class II molecules, and their activation is influenced by various factors, including the cell cycle and external stimuli such as interferon-gamma (IFN-γ). This response is critical in controlling and maintaining the immune response, and its deficiency can lead to severe immunodeficiency.
| Characteristics | Values |
|---|---|
| MHC Class II molecules | Heterodimeric glycoproteins composed of an α and a β chain |
| MHC Class II functions | Presenting extracellular pathogens to immune cells like T helper cells (CD4+) |
| MHC Class II expression | Tightly regulated in all APCs both transcriptionally and post-transcriptionally |
| MHC Class II transactivator | CIITA, the master regulator of MHC Class II gene expression |
| CIITA expression | Constitutive in professional APCs like macrophages |
| MHC Class II activation | Induced by IFN-γ, with maximal induction 24-48 hours after stimulation |
| MHC Class II deficiency | Can lead to bare lymphocyte syndrome and immunodeficiency |
| MHC Class II ubiquitination | Regulated by March-I, with IL-10 up-regulating March-I expression and MHC-II ubiquitination |
Explore related products
What You'll Learn

MHC Class II molecules are heterodimeric glycoproteins
MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules. MHC Class II molecules are heterodimeric glycoproteins composed of an α and a β chain, which are displayed at the surface of professional antigen-presenting cells (APCs). MHC Class II molecules are normally found only on professional APCs such as dendritic cells, macrophages, some endothelial cells, thymic epithelial cells, and B cells.
The α and β chains of MHC Class II molecules are produced during synthesis in the endoplasmic reticulum. These chains are complexed with a special polypeptide known as the invariant chain. The invariant chain is a fragment of a larger protein, the Class II-associated invariant chain, which is involved in the loading of antigens onto MHC Class II molecules. The invariant chain is removed from the MHC Class II molecule by the enzyme HLA-DM, allowing for peptide binding to occur.
APCs play a crucial role in the immune system by presenting antigens to T cells. MHC Class II molecules present antigens of exogenous origin, specifically extracellular pathogens, to CD4+ T cells. These antigens are acquired through phagocytosis and undergo endosomal degradation to generate peptides that are loaded onto MHC Class II molecules. The peptide-MHC Class II complex is then recognized by the cognate T cell receptor (TCR) of helper T cells, leading to the activation of these lymphocytes.
The expression of MHC Class II molecules is tightly regulated in APCs through mechanisms such as transcriptional and post-transcriptional control. MHC Class II transactivator (CIITA) is the master regulator of MHC Class II gene expression and is constitutively expressed by professional APCs. However, CIITA activity and MHC Class II expression can be influenced by factors such as interferon-γ (IFNγ), which can induce the conversion of monocytes into MHC Class II-expressing APCs.
Judicial Power: Democracy in the US Constitution?
You may want to see also

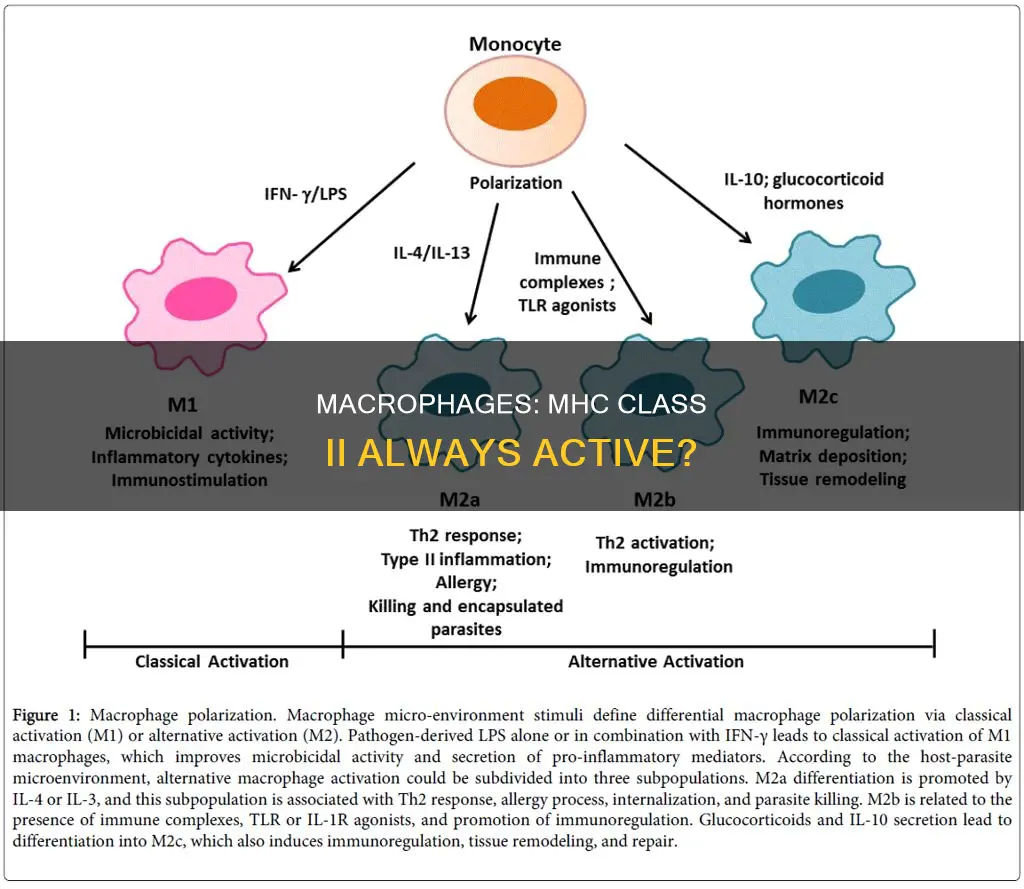
Macrophages are activated by IFN-γ or LPS
Macrophages are versatile cells that can assume diverse roles in response to various stimuli. Macrophage activation is synergistically controlled by interferon-gamma (IFN-γ) and lipopolysaccharide (LPS).
IFN-γ, a cytokine produced by T-helper cells, promotes macrophage activation by activating STAT1-dependent genes and suppressing STAT3-dependent negative feedback regulation downstream of LPS signalling. This process primes macrophages and enhances their activation through mechanisms such as chromatin remodelling and metabolic reprogramming. Specifically, IFN-γ induces a rapid shift from oxidative phosphorylation to aerobic glycolysis in M1 macrophages, sustaining cell viability and inflammatory activity.
LPS, a bacterial endotoxin, also plays a critical role in macrophage activation. Classical activation of M1 macrophages with LPS induces a metabolic switch, enhancing glycolytic flux and reducing oxidative metabolism. This metabolic remodelling is associated with macrophage activation and the development of inflammatory diseases such as atherosclerosis.
The activation of macrophages by IFN-γ and LPS has significant implications for tumour biology. Tumour-associated macrophages can either promote or suppress tumour growth depending on their activation status. IFN-γ is identified as a crucial factor in inducing the tumoricidal M1 phenotype in macrophages, characterised by direct cytostatic and cytotoxic effects on tumour cells. However, there is ongoing debate about whether IFN-γ alone is sufficient, or if additional stimuli, such as LPS, are required to fully activate macrophages and induce their tumoricidal capabilities.
In summary, macrophages are activated by IFN-γ and LPS through synergistic mechanisms that involve gene regulation, metabolic reprogramming, and immunomodulation. These activated macrophages play essential roles in host defence, inflammatory diseases, and tumour suppression.
Who Really Wrote the Constitution?
You may want to see also

MHC Class II presents antigens to T cells
MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules that are found on professional antigen-presenting cells (APCs) such as dendritic cells, macrophages, some endothelial cells, thymic epithelial cells, and B cells. These cells play a crucial role in initiating immune responses by presenting antigens to T cells.
The antigens presented by MHC Class II molecules are exogenous, originating from extracellular proteins rather than cytosolic and endogenous sources like those presented by MHC Class I. The process begins with the acquisition of extracellular pathogens through phagocytosis. These pathogens are then broken down in a lysosome, and specific components are loaded onto MHC Class II molecules.
The MHC Class II molecule then migrates to the cell surface, where it presents the antigen to a helper T cell. This interaction activates the T cell, leading to the release of cytokines and other molecules that aid in combating pathogens outside the cells. This process is essential for the induction and regulation of adaptive immunity, as it selects the mature CD4+ T cell repertoire in the thymus and activates these lymphocytes in the periphery.
The ability of MHC Class II molecules to present antigens to T cells is crucial for overall immune function. The stability of peptide binding ensures that the antigen remains attached to the MHC molecule, allowing for T cell recognition and a proper immune response. Deficient MHC Class II molecules can lead to a decrease in T cell population, impacting the activation of B cells and the overall immune response cascade.
The expression of MHC Class II molecules is tightly regulated in APCs through transcriptional and post-transcriptional mechanisms. MHC Class II transactivator (CIITA) is the key regulator of MHC Class II gene expression, and its activity can be influenced by interferon-γ (IFNγ), which can convert monocytes into MHC Class II-expressing APCs.
Georgia's Constitutional Evolution: Multiple Iterations Explored
You may want to see also
Explore related products

MHC Class II activates helper T cells
MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules found on the surface of professional antigen-presenting cells (APCs). These include dendritic cells, macrophages, some endothelial cells, thymic epithelial cells, and B cells. MHC Class II molecules play a crucial role in initiating immune responses by presenting antigens to helper T cells.
The process begins with the acquisition of extracellular pathogens through phagocytosis. These pathogens are then broken down in a lysosome, and specific components are loaded onto MHC Class II molecules. The MHC Class II molecule then migrates to the cell surface to present the antigen to a helper T cell. This presentation of the antigen to the helper T cell is a critical step in activating the helper T cell.
The activation of helper T cells by MHC Class II molecules triggers the release of cytokines and other molecules that help induce and activate other cells to combat extracellular pathogens. This activation is facilitated by the interaction between the peptide-MHC Class II complex and the cognate T cell receptor (TCR) of helper T cells. This interaction is essential for the induction and regulation of adaptive immunity, as it shapes the mature CD4+ T cell repertoire in the thymus and activates these lymphocytes in the periphery.
The activation of helper T cells also has downstream effects on other immune cells, such as B cells. Once activated, helper T cells can activate specific B cells that display the same complex of foreign peptide and MHC Class II protein on their surface. This activation of B cells leads to their division, proliferation, and differentiation into plasma cells, which are responsible for producing antibodies to combat the infection.
The proper functioning of MHC Class II molecules is vital for the overall immune response. Deficient MHC Class II molecules can lead to a decrease in T cell numbers and impair the activation of both T cells and B cells, disrupting the immune response cascade. Therefore, MHC Class II molecules play a central role in activating helper T cells and coordinating the immune system's response to extracellular pathogens.
Salesperson Conduct: Fraud or Not?
You may want to see also

MHC Class II deficiency can cause bare lymphocyte syndrome
MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules found on professional antigen-presenting cells (APCs) such as dendritic cells, macrophages, endothelial cells, thymic epithelial cells, and B cells. These cells are crucial in initiating immune responses by presenting antigens to CD4+ T cells. MHC Class II deficiency, also known as bare lymphocyte syndrome, is a rare autosomal recessive primary immunodeficiency caused by mutations in the genes that regulate MHC Class II expression. This results in a depletion of CD4+ T cells, impaired antigen presentation, and a compromised immune response.
Bare lymphocyte syndrome type II (BLS type II) or MHC Class II deficiency is characterized by a severe decrease in HLA class II molecules, leading to immunodeficiency. Affected individuals present in early infancy with severe recurrent infections, typically involving the gastrointestinal and respiratory tracts. Infections are caused by bacteria, viruses, fungi, and protozoa, and are often accompanied by protracted diarrhea and failure to thrive. About 20% of patients develop autoimmune features, and laboratory studies reveal reduced CD4+ T cell counts, abnormal lymphocyte proliferation, and hypogammaglobulinemia.
The genetic lesions responsible for MHC Class II deficiency occur in the transacting regulatory genes required for MHC Class II gene transcription. Several genes have been implicated, including RFXANK, RFX5, RFXAP, and CIITA. These genes encode transcription factors that regulate MHC Class II expression. The abnormal expression of HLA molecules is due to defective synthesis, which is secondary to mutations in these regulatory genes.
The deficiency of MHC Class II molecules impairs the activation and proliferation of T cells and disrupts the immune response cascade, including the function of B cells. This results in a decreased number of T cells, preventing their interaction and activation of B cells. Normally, activated B cells divide, proliferate, and differentiate into plasma cells. However, in MHC Class II deficiency, this process is hindered, further compromising the immune response.
Therapy for bare lymphocyte syndrome type II aims to reduce infections and control infection through the administration of antibiotics and Ig replacement therapy. Hematopoietic stem cell transplantation (HSCT) can resolve the immune deficiency, with a higher chance of success if performed within the first two years of life. Without HSCT, individuals with MHC Class II deficiency have a high mortality rate, often succumbing to overwhelming infections by the age of four.
Death Penalty: Constitutional Support Explained
You may want to see also
Frequently asked questions
MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules that are found on professional antigen-presenting cells (APCs) such as dendritic cells, macrophages, some endothelial cells, thymic epithelial cells, and B cells.
MHC Class II molecules present antigens of exogenous origin to CD4+ T cells. These antigens undergo phagocytosis and endosomal degradation to generate peptides that are loaded onto MHC Class II molecules. The peptide:MHC Class II complex is then recognized by the cognate T cell receptor (TCR) of helper T cells.
MHC Class II is not constitutively active on macrophages. Macrophages express low levels of MHC Class II molecules under basal conditions, and they must be activated to induce the expression of MHC Class II molecules. Interferon-gamma (IFN-γ) is the major macrophage activator that induces MHC Class II expression.











































