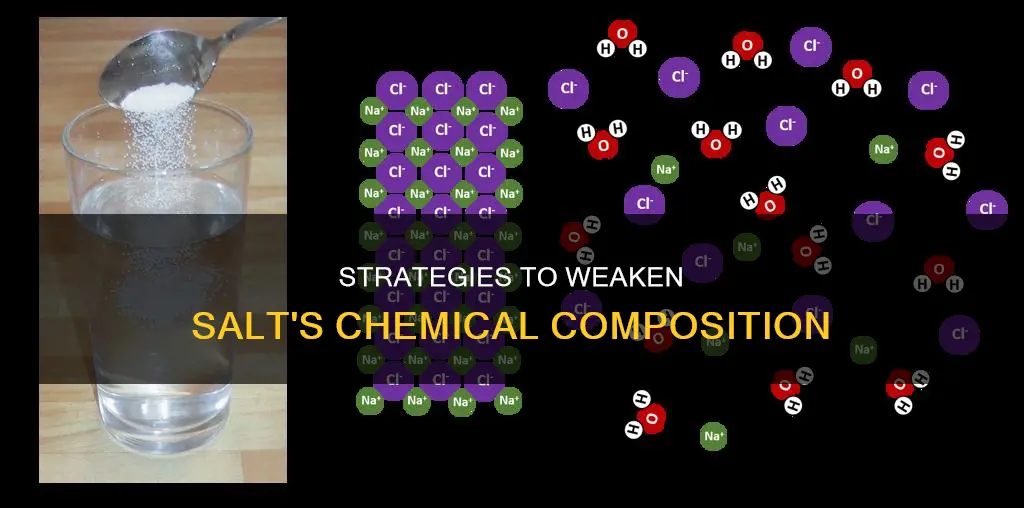
Salt is a mineral composed primarily of sodium chloride (NaCl). In chemistry, a salt or ionic compound is a chemical compound consisting of an assembly of positively charged ions (cations) and negatively charged ions (anions). The constituent ions are held together by electrostatic forces termed ionic bonds. One way to weaken the chemical constitution of salt is to dissolve it in water. The negatively charged oxygen atoms of water are drawn to the positively charged sodium ions, and the positively charged hydrogen atoms of water are attracted to the negatively charged chloride ions. This attraction weakens the ionic bonds within the sodium chloride crystal lattice. This process is called hydration, and it stabilizes the separated ions.
| Characteristics | Values |
|---|---|
| Chemical composition | Ionic compound consisting of positively charged ions (cations) and negatively charged ions (anions) |
| Electrical properties | Electrically neutral, but becomes conductive when dissolved in water |
| Solubility | Dissolves in water, breaking down into ions (sodium and chloride) |
| Hydration | Water molecules surround and stabilize individual ions through hydration |
| Structure | Alternating large negative anions and small positive cations form a crystal lattice |
| Sources | Seawater, salt mines, mineral-rich spring water, sedimentary deposits |
| Uses | Food seasoning, fertilizer, water treatment, chemical production, de-icing, industrial processes |
Explore related products
$19.97 $24.99
What You'll Learn

Breaking the ionic bonds in the salt crystal
Salt, or a chemical compound consisting of positively charged ions (cations) and negatively charged ions (anions), is held together by electrostatic forces called ionic bonds. These ionic bonds can be weakened or broken by introducing a solvent, such as water, that has a stronger attraction for the ions than their mutual attraction.
When salt is mixed with water, the covalent bonds of water are stronger than the ionic bonds in the salt molecules. The positive side of the water molecules is attracted to the negative chloride ions, while the negative side of the water molecules is attracted to the positive sodium ions. This creates a competition in which the water molecules ultimately win, pulling the sodium and chloride ions apart and breaking the ionic bond.
The process of dissolving salt in water can be understood through the concept of electrical charges. Both water and salt compounds are polar, with positive and negative charges on opposite sides of the molecule. The chloride ion in salt is negatively charged, while the sodium ion is positively charged. Similarly, a water molecule is also ionic, with two hydrogen atoms positioning their positive charge on one side of the oxygen atom, which carries a negative charge.
Additionally, the mobility of ions in salt can be influenced by temperature. In some cases, such as in fast-ion conductors and ionic glasses, certain ionic components have significant mobility, allowing conductivity even when the material is solid. This temperature-dependent behaviour may result from a phase change or a high defect concentration. These materials are commonly used in solid-state supercapacitors, batteries, fuel cells, and chemical sensors.
It is worth noting that while water is often referred to as the "universal solvent," it cannot dissolve every substance. However, it does dissolve more substances than any other liquid, making it a versatile solvent with significant implications for life on Earth.
Separation of Powers: Constitution's Definition and Purpose
You may want to see also

Neutralising the positive and negative ions
Salt, or a salt compound, is a chemical compound consisting of an assembly of positively charged ions (cations) and negatively charged ions (anions). These ions are held together by electrostatic forces, termed ionic bonds. The ions form a lattice structure, with each sodium ion (Na+) being surrounded by chloride ions (Cl-) and vice versa.
To weaken the chemical constitution of salt, one must disrupt the balance of these positive and negative ions. This can be achieved by dissolving the salt in a polar liquid such as water. When salt is dissolved in water, the water molecules help to dismantle the salt crystal. This is because the partial positive and negative charges on the polar water molecules provide a substitute for the positive and negative charges that were present in the crystal lattice. Each positively charged Na+ is surrounded by water molecules with their negatively charged oxygens turned toward it, and each negatively charged Cl- ion is surrounded by water molecules with their positively charged hydrogens closest. This process is known as hydration, and it results in the salt ions being stabilised by the water molecules.
The stability of the hydrated ions in solution must be greater than the stability of the crystal lattice for the salt to dissolve. This is because the electrostatic hydration energy provided by the water molecules needs to compensate for the loss of attractions between ions in the salt crystal.
In summary, to weaken the chemical constitution of salt, one can dissolve the salt in water, taking advantage of the polar nature of water molecules to disrupt the balance of positive and negative ions in the salt crystal lattice. This process of hydration weakens the ionic bonds that hold the salt compound together, resulting in a weakened chemical constitution of the salt.
The Constitution: A Self-Serving Document?
You may want to see also

Using water to dissolve the salt
Salt, or sodium chloride (NaCl), is a soluble ionic compound, meaning it will dissolve to the point where it is no longer visible. When dissolved in water, the sodium chloride compound breaks down into its constituent ions, Na+ and Cl-. The positive and negative polar ends of water molecules attract the negative chloride ions and positive sodium ions in the salt, respectively. This process is facilitated by the presence of more water molecules, higher water temperatures, and minimal impurities in the water and salt.
To dissolve salt in water, first determine the amount of salt you intend to use and pour it into a labelled container. Then, pour the desired amount of water into the container. If the salt does not immediately dissolve, you can stir it with a spoon or spatula to facilitate the process. Warmer water will dissolve more salt than cooler water, so you can heat the mixture to help the salt dissolve. Additionally, using distilled or deionized water can minimize contamination and provide more water molecules to interact with the salt.
The solubility of a substance refers to the amount that can dissolve in a liquid at a specific temperature. Salt is highly soluble in water, and the resulting solution exhibits altered properties compared to pure water or salt alone. For example, the freezing point of water is lowered when salt is dissolved in it. However, the solution still consists of water molecules and salt; no new molecules or bonds are formed.
It is important to note that the presence of other solutes in the water can impact the dissolution process. The more solutes present, the fewer water molecules are available to interact with the salt. Additionally, there may be interactions between the solutes and the salt, depending on their nature. Therefore, it is recommended to use distilled or deionized water to minimize contamination and ensure consistent results.
By understanding the variables that affect the dissolution of salt in water, such as temperature, purity, and the presence of other solutes, you can effectively utilize water to weaken the chemical constitution of salt by breaking down the sodium chloride compound into its individual ions.
Protein Power: How Much Is Too Much?
You may want to see also
Explore related products

Changing the temperature to impact the salt's structure
The dissolution of salt is a process that can be either endothermic or exothermic, depending on the type of salt and the solution. In an endothermic reaction, the system absorbs heat from its surroundings, resulting in a decrease in temperature. Conversely, in an exothermic reaction, the system releases heat into its surroundings, leading to an increase in temperature.
Let's consider an example where salt is dissolved in water. Initially, both the water and the salt are at the same temperature. However, upon dissolving the salt, the temperature of the water may change. This temperature change is due to the energy exchange that occurs during the breaking and forming of bonds in the dissolution process.
The solubility of most solutes, including some salts, increases with temperature. As the temperature rises, the molecules of the solvent gain more kinetic energy, causing them to move randomly and creating greater distances between them. This results in larger voids that provide more space for the solute molecules to fit in, thus increasing the solubility.
However, there are exceptions to this trend. Some salts, such as cerium sulphate, lithium carbonate, and sodium carbonate monohydrate, exhibit decreased solubility as the temperature increases. This unusual behaviour can be explained by the enthalpy of dissolution. Typically, the energy required to break the interactions in the solid is expected to be higher than the energy gained from solvating the solute. However, in these cases, the opposite seems to be true, indicating a more complex relationship between temperature and solubility for certain salts.
To investigate the impact of temperature on salt structure and solubility, a laboratory experiment can be designed using a coffee-cup calorimeter. By dissolving different salts, such as ammonium nitrate, calcium chloride, magnesium carbonate, and sodium chloride, in water at various temperatures, students can observe and record temperature changes and analyse the enthalpies of dissolution. This hands-on approach provides valuable insights into the complex behaviour of different salts in response to temperature variations.
Understanding the Plantar Region: Heel Inclusion Explored
You may want to see also

Using salt in water to melt ice
Salt is a chemical compound consisting of positively charged ions (cations) and negatively charged ions (anions). These ions are held together by electrostatic forces called ionic bonds. An example of a salt is sodium chloride (NaCl), which is commonly used to melt ice on roads.
When salt is added to water, it lowers the freezing point of the water, which is why salt is used to melt ice. The freezing point of pure water is 32 degrees Fahrenheit (0 degrees Celsius), but when salt is added, the freezing temperature decreases to around 0 degrees Fahrenheit (minus 18 degrees Celsius). This is because the salt disrupts the equilibrium of the ice-water mixture, allowing it to remain in a liquid state at lower temperatures.
To use salt and water to melt ice, you can create a salt water solution by dissolving salt in water. This solution can then be sprayed or poured onto the ice. Alternatively, you can spread solid rock salt on the ice, and then add water, which will dissolve the salt and form a solution on the ice. This will lower the freezing point of the ice, causing it to melt.
It is important to note that rock salt has limitations and environmental impacts. If the temperature is lower than 15 degrees Fahrenheit (minus 9 degrees Celsius), rock salt will not be effective in melting ice. Additionally, the sodium and chlorine in rock salt can leach into the ground and water, affecting plants, aquatic animals, and wetlands. Therefore, it is essential to use salt sparingly and consider alternative methods or substances, such as sand, molasses, beet juice, or pickle juice, to prevent slipping and reduce environmental harm.
Framers' Vision: Democracy and the Constitution
You may want to see also
Frequently asked questions
The negatively charged oxygen atoms of water are attracted to the positively charged sodium ions, and the positively charged hydrogen atoms of water are attracted to the negatively charged chloride ions. This attraction weakens the ionic bonds within the sodium chloride crystal lattice, a process known as hydration. The water molecules then surround each individual ion, stabilizing them in the aqueous environment.
Salt is an ionic chemical compound consisting of positively charged ions (cations) and negatively charged ions (anions). The constituent ions are held together by electrostatic forces termed ionic bonds. The component ions in a salt can be either inorganic, such as chloride (Cl−), or organic, such as acetate (CH3COO−).
Weak salts or weak electrolyte salts are composed of weak electrolytes and do not dissociate well in water. They are generally more volatile than strong salts and may have a similar odor to the acid or base they are derived from. An example of a weak salt is sodium acetate, which smells similar to acetic acid.



![[1 Gallon] Concentrated Salt Remover + Corrosion Protection - Made in USA, Salt Cleaner Ideal for Boats, Cars, Marine Engine & Outboard Motor Flush, Washes Salt Away from Boat, Vehicles, & Trailers](https://m.media-amazon.com/images/I/712KKplfhDL._AC_UL320_.jpg)





![[1 Gallon] Salt Remover Concentrate - Marine Engine Flush for Boats - Perfect for Outboard Motor Flush & Washing Salt Deposits - Remove Salt Deposits for Autos](https://m.media-amazon.com/images/I/81UCevJQTFL._AC_UL320_.jpg)