When identifying the number of pi bonds in constitutional isomers, it is important to understand the difference between sigma and pi bonds. Sigma bonds are single covalent bonds, while double covalent bonds consist of one sigma bond and one pi bond. To identify the number of pi bonds, count the number of double bonds in the molecule. For example, a double bond consists of one sigma bond and one pi bond, while a triple bond consists of one sigma bond and two pi bonds. Additionally, there are formulas to calculate the number of double bond equivalents (DBE) in a molecule, which can help identify the presence of pi bonds.
| Characteristics | Values |
|---|---|
| Single covalent bonds | Sigma bonds |
| Double covalent bonds | One sigma bond and one pi bond |
| Triple covalent bonds | Two pi bonds |
| Formula to determine the number of constitutional isomers | Longest chain or ring to shortest chain or ring |
Explore related products
What You'll Learn

Double covalent bonds have one pi bond
A double covalent bond is composed of one sigma bond and one pi bond. Sigma bonds are formed by the end-to-end overlap of orbitals, with the electron density concentrated between the nuclei of the bonding atoms. On the other hand, pi bonds are formed by the side-by-side overlap of orbitals, with the electron density concentrated above and below the plane of the nuclei of the bonding atoms.
In a double covalent bond, the sigma bond is the first bond formed between the atoms, while the pi bond is the "second" bond. For example, in the molecule ethene (C₂H₄), there is a double covalent bond between the two carbon atoms, with single bonds between the carbon atoms and the hydrogen atoms. The entire molecule is planar.
The presence of a double bond can be indicated in Lewis electron-dot structures by a double dash (=) between the atoms, as in C=C. This indicates that there are two electron pairs involved in the double bond.
Pi bonds are typically weaker than sigma bonds due to less overlap between the component p-orbitals because of their parallel orientation. However, the combination of a pi bond and a sigma bond results in a stronger bond than either type of bond by itself.
When considering constitutional isomers, it is important to note that the presence of double bonds can affect the number of possible isomers. For example, in the case of butane, you can add double bonds to form different configurations of isomers. By following a systematic approach, such as starting with the longest chain or ring and adding double bonds accordingly, you can explore various configurations and identify the possible isomers.
US vs Russia: Constitutions Compared
You may want to see also

Triple covalent bonds have two pi bonds
In chemistry, covalent bonds are a type of chemical bond formed when two atoms share electrons. There are two types of covalent bonds: sigma bonds and pi bonds. Sigma bonds are formed by the overlap of orbitals in an end-to-end fashion, with the electron density concentrated between the nuclei of the bonding atoms. On the other hand, pi bonds are formed by the overlap of orbitals in a side-by-side fashion, with the electron density concentrated above and below the plane of the nuclei of the bonding atoms.
Now, let's focus on triple covalent bonds and their pi bonds. A triple covalent bond is a type of chemical bond in which three pairs of electrons are shared between two atoms. In the case of triple covalent bonds, there are indeed two pi bonds present. This can be understood by examining molecules such as ethyne (C2H2), which features a triple bond between two carbon atoms.
The hybridization of orbitals in ethyne involves the 2s orbital and the 2px orbital, while the 2py and 2pz orbitals remain unhybridized. The sp hybrid orbitals form a strong sigma bond between each other, as well as sigma bonds to any hydrogen atoms. The remaining unhybridized orbitals (2py and 2pz) then form two pi bonds, positioned side-by-side with electron density above and below the molecular plane.
The presence of two pi bonds in a triple covalent bond is a fundamental concept in chemistry. It is important to distinguish between sigma and pi bonds, as they contribute differently to the overall bond strength and molecular geometry. The knowledge of these bond types is essential for understanding the structure and reactivity of various chemical compounds.
In summary, triple covalent bonds indeed possess two pi bonds. This is a result of the orbital hybridization and side-by-side overlap of unhybridized orbitals, forming the characteristic electron density distribution associated with pi bonds.
The Constitution's Long-Standing Legacy
You may want to see also

A formula to calculate the number of double bond equivalents (DBE)
The double bond equivalent (DBE) is a representation of the number of pi bonds or rings in an organic molecule. It is a measure of the degree of unsaturation of a molecule. The DBE formula is used to calculate the number of double bond equivalents and is given by:
DBE = Number of Carbon atoms + Number of Hydrogen atoms + Number of Halogen atoms + Number of Nitrogen atoms
Alternatively, the DBE can be calculated using the formula:
DBE = Number of Carbon atoms + Number of Hydrogen or Halogen atoms + Number of Nitrogen or Phosphorus atoms
It is important to note that the presence or absence of oxygen atoms does not impact the DBE value. Additionally, a double bond is equivalent to 1 DBE, a triple bond is equivalent to 2 DBE, and a ring is equivalent to 1 DBE.
For example, let's calculate the DBE for the molecule glucose, which has the formula C6H12O6. Since oxygen does not impact the DBE, we can ignore it and consider only carbon and hydrogen atoms. Substituting the values into the equation, we get:
DBE = 6 + 12
Simplifying the equation, we find that the DBE value for glucose is 18.
The Confederate Constitution: Preamble Differences
You may want to see also
Explore related products

Using the longest chain or ring to the shortest method
To determine the number of pi bonds in constitutional isomers using the longest chain or ring to the shortest method, one must first identify the number of carbon atoms (represented as 'X') and the number of hydrogen atoms (represented as 'Y') in the given unsaturated hydrocarbon containing double bonds. This information is crucial for applying the appropriate formulae to calculate the number of pi bonds.
For aliphatic straight-chain olefins, the formula to calculate the number of pi bonds (P) is given by the equation: P = (2X - Y)/2 + 1. By substituting the values of X and Y into this equation, you can determine the number of pi bonds.
On the other hand, for aliphatic cyclic olefins, the formula to calculate the number of pi bonds (Pc) differs slightly. It is given by the equation: Pc = (2X - Y)/2. Again, by plugging in the values of X and Y into this equation, the number of pi bonds can be ascertained.
It is important to note that these formulae are applicable when dealing with unsaturated hydrocarbons containing double bonds. The presence of double bonds is a key factor in determining the number of pi bonds within a molecule.
Additionally, this method can also be used to calculate the number of sigma bonds (single bonds) in both straight-chain and cyclic olefinic hydrocarbons. The formula for aliphatic straight-chain olefins is given by: S = X + Y - 1, while for aliphatic cyclic olefins, it is: Sc = X + Y - 2.
By following these systematic steps and applying the appropriate formulae based on the type of hydrocarbon and the presence of double bonds, one can effectively determine the number of pi bonds in constitutional isomers using the longest chain or ring method.
Drinking Habits: When Does It Become Alcoholism?
You may want to see also

Counting the number of double bonds
While there is no simple method to determine the number of constitutional isomers in a molecule with multiple double bonds, there are some mathematical formulae and patterns that can help. For example, the formula for the maximum number of configurational stereoisomers (not constitutional isomers) is $N_\text{max}=2^{\left(n+m\right)}$, where $n$ is the number of stereocentres (R or S) and $m$ is the number of stereogenic double bonds (E or Z).
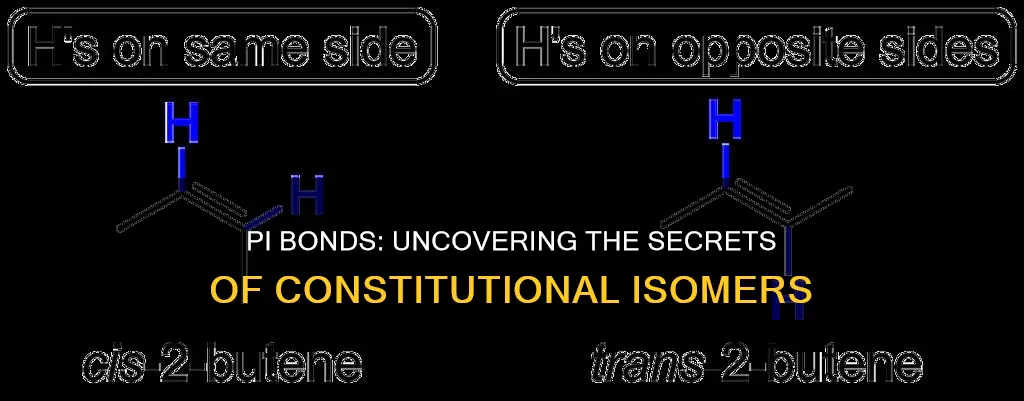
Constitutional isomers are defined as compounds that have the same molecular formula but different structures. Stereoisomers, on the other hand, are isomers in which the atoms are connected in the same way but differ in the way the atoms are arranged in space. Cis/trans isomers, such as cis- and trans-2-butene, are examples of stereoisomers. The presence of a double bond restricts rotation, so alkenes form stereoisomers that differ in how substituents are arranged around the double bond.
The geometry around the C=C double bond in an alkene is important in the chemistry of these compounds. The sigma bond skeleton in a C2H4 molecule is formed by the overlap of sp2 hybridized orbitals on each carbon atom with either a 1s orbital on a hydrogen atom or the sp2 hybridized orbital on the other carbon atom. This leaves one unpaired electron in an empty 2p orbital on each carbon atom, and the orbitals that hold these electrons interact to form a bond.
There is also a formula to work out the number of double bond equivalents (DBE): a double bond equals 1 DBE, a triple bond equals 2 DBE, and a ring equals 1 DBE. For example, the formula for butene, C4H8, indicates that all the isomers must incorporate either a ring or a double bond.
American Constitution: Truly Democratic?
You may want to see also
Frequently asked questions
Pi bonds occur in double and triple covalent bonds. Double covalent bonds have one pi bond, and triple covalent bonds have two pi bonds.
Count the number of double and triple bonds in the molecule. The number of double bonds is equal to the number of pi bonds, and each triple bond contributes two pi bonds.
Yes, there is a formula to calculate the number of double bond equivalents (DBE). Double bond = 1 DBE, Triple Bond = 2 DBE, and Ring = 1 DBE.











































