Constitutional isomers are compounds with the same molecular formula but different atomic connectivity. They are also known as structural isomers. The easiest way to determine if molecules are constitutional isomers is to count the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index). If all atoms are the same and molecules have the same HDI, they are constitutional isomers. However, to be absolutely sure, it is important to name the molecules according to IUPAC nomenclature rules. Constitutional isomers can have different functional groups, carbon backbones, and bonding patterns, and they can be differentiated from stereoisomers, which have the same connectivity but differ in the arrangement of atoms in space.
| Characteristics | Values |
|---|---|
| Molecular formula | Same |
| Number of atoms | Same |
| Connectivity of atoms | Different |
| Number of carbons | Same |
| Degree of unsaturation | Same |
| Hydrogen Deficiency Index (HDI) | Same |
| Structural motifs | Different |
| Functional groups | Same or different |
| Physical and chemical properties | Different |
Explore related products
What You'll Learn
- Constitutional isomers have the same molecular formula but different bonding patterns
- To determine isomers, count the number of carbons and the degree of unsaturation
- Compare the number of atoms and the net charge
- Constitutional isomers can have different functional groups
- Stereoisomers have the same connectivity but differ in the arrangement of atoms

Constitutional isomers have the same molecular formula but different bonding patterns
Constitutional isomers are compounds with the same molecular formula but different bonding patterns. They are also known as structural isomers. The key difference between constitutional isomers is how the atoms are connected, or their "connectivity".
For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers with the molecular formula C2H6O. They have the same atoms in the same ratios, but the connections between those atoms are different. In ethanol, the atomic connectivity is C—C—O, with the oxygen atom being part of an alcohol. In dimethyl ether, the connectivity is C—O—C, forming an ether.
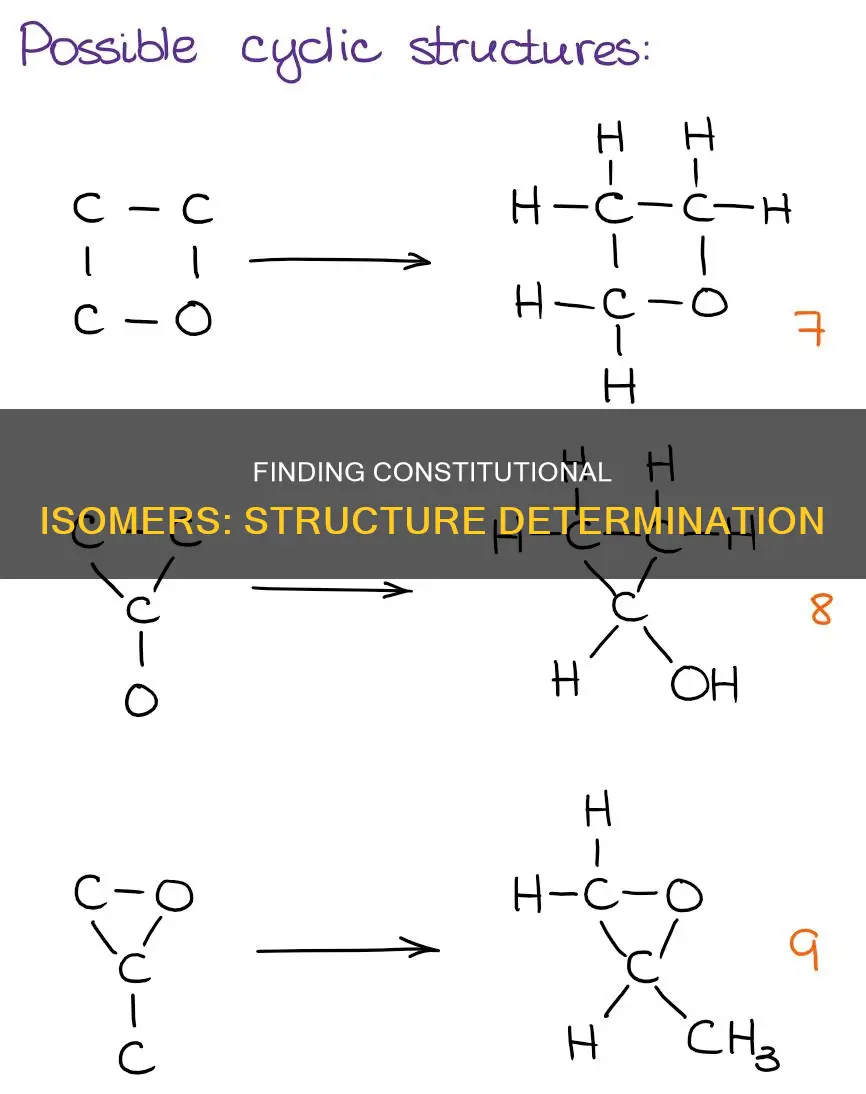
Another way to determine if molecules are constitutional isomers is to count the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index or HDI). If all the atoms are the same and the molecules have the same HDI, then they are likely constitutional isomers. The HDI can be calculated using a simple formula and can help in drawing various constitutional isomers with the correct structural motifs.
Constitutional isomers can also be identified by their different functional groups. For instance, 1-propanol and 2-propanol are constitutional isomers with the same molecular formula, but their hydroxyl group is attached to different carbon atoms.
In summary, constitutional isomers have the same molecular formula but differ in their bonding patterns, atomic connectivity, and functional group placement. These differences can lead to distinct physical and chemical properties, making it important to be able to identify and distinguish between constitutional isomers.
Expert Reports: Are They Evidence in New York?
You may want to see also

To determine isomers, count the number of carbons and the degree of unsaturation
To determine whether molecules are constitutional isomers, you can start by counting the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index). If the molecules have the same number of atoms and the same HDI, they are likely constitutional isomers. However, this method may not be sufficient for large molecules, and naming the molecules according to IUPAC nomenclature rules may be necessary to be absolutely sure.
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different connectivity. In other words, they have the same types and numbers of atoms but differ in the way these atoms are connected to one another. For example, ethanol (drinking alcohol) and dimethyl ether have the same formula, C2H6O, but different atomic connectivities: C—C—O in ethanol and C—O—C in dimethyl ether.
The number of possible structural isomers increases rapidly with the number of carbons, making it challenging to identify all isomers by hand. For instance, there are 75 isomers and 136 stereoisomers for molecules with 10 carbons. This relationship between the number of carbons and the number of possible isomers has been an interesting research topic in computational chemistry and mathematics.
The degree of unsaturation of a molecule can be calculated by determining the number of rings and/or multiple bonds present. For example, the formula C5H8O has two degrees of unsaturation compared to its equivalent hydrocarbon formula C5H8. Similarly, organohalogen compounds can be converted to their equivalent hydrocarbon formulae by adding the number of halogens and hydrogens, allowing for the calculation of the degree of unsaturation.
The Future of Governance: Constitution or Communist Manifesto?
You may want to see also

Compare the number of atoms and the net charge
Constitutional isomers are compounds with the same formula but different connectivity. In other words, constitutional isomers have the same number of atoms and the same net charge, but the atoms are connected differently. For example, ethanol and dimethyl ether both have the molecular formula C2H6O, but they have completely different physical and chemical properties due to the different ways their atoms are connected.
The easiest way to determine if molecules are constitutional isomers is to count the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index). If all the atoms are the same and the molecules have the same HDI, then they are constitutional isomers. However, it is important to note that molecules can be drawn in different ways, and just because they look different does not mean they are different compounds. For example, two molecules may look different but could simply represent a rotation around a single bond.
To be absolutely sure that two molecules are constitutional isomers, it is necessary to name the molecules according to the IUPAC nomenclature rules. Additionally, constitutional isomers can have the same or different functional groups. As long as the molecular formula is the same, they are considered constitutional isomers. For example, ethanol and dimethyl ether have the same molecular formula (C2H6O), but their functional groups differ. Ethanol has a C—C—O connectivity, while the isomer forms an ether with C—O—C connectivity.
In summary, when comparing the number of atoms and the net charge to determine if molecules are constitutional isomers, it is important to remember that constitutional isomers have the same number of atoms and the same net charge but differ in the way the atoms are connected. To identify constitutional isomers, one can count the number of carbons and the degree of unsaturation, keeping in mind that molecules can adopt different conformations due to free rotation around single bonds.
The Constitution and Indigenous Americans: A Complex History
You may want to see also
Explore related products

Constitutional isomers can have different functional groups
To determine whether molecules are constitutional isomers, it is necessary to compare their chemical formulae and their degree of unsaturation (Hydrogen Deficiency Index). If the molecules have the same chemical formula and the same HDI, they are constitutional isomers. However, it is important to remember that molecules are flexible and can be drawn in multiple ways, so this visual comparison is not always sufficient.
Constitutional isomers are compounds with the same formula but different connectivity. That is, constitutional isomers have the same types and numbers of atoms, but these atoms are connected differently. For example, ethanol and dimethyl ether have the same molecular formula, C2H6O, but their atomic connectivity is different. In ethanol, the atomic connectivity is C—C—O, and the oxygen atom is part of an alcohol. In dimethyl ether, the connectivity is C—O—C, and the oxygen atom forms an ether.
An important class of constitutional isomers is positional isomers, in which the functional groups are the same but differ in their location within the molecule. For example, the isomers 6 and 7, or the ortho, meta, and para isomers.
Civil Servants: Sworn to Protect the Constitution?
You may want to see also

Stereoisomers have the same connectivity but differ in the arrangement of atoms
When it comes to the topic of how to determine the different structures of a constitutional isomer, it's important to understand the concept of isomers and their categories. Isomers are compounds that have the same molecular formula but differ in their internal structure, particularly in the way atoms are connected. These differences in connectivity can lead to distinct physical and chemical properties.
Constitutional isomers, also known as structural isomers, are a type of isomer that falls into one of the two primary categories. They are defined by having the same molecular formula but different connectivities. In other words, the atoms are linked in unique ways, resulting in variations in their carbon backbones or functional groups. For instance, ethanol (drinking alcohol) and dimethyl ether share the formula C2H6O but exhibit distinct properties due to their distinct atomic connections.
Now, let's delve into stereoisomers, which are relevant to your specific focus. Stereoisomers are isomers that share the same connectivity but differ in the arrangement of their atoms in space. They have identical bonding structures but exhibit variations in the geometric positions of their functional groups and atoms. This means that stereoisomers can have the same semi-expanded formula but differ in their spatial geometry.
To visualize stereoisomers, consider the following examples: cis-1,2-dibromocyclobutane and trans-1,2-dimethylcyclopentane. In cis-1,2-dibromocyclobutane, the bromine atoms are on the same side of the cyclobutane ring. On the other hand, in trans-1,2-dimethylcyclopentane, the two methyl (CH3) groups are positioned on opposite sides of the ring. These compounds, with their distinct spatial arrangements, help illustrate the concept of stereoisomers.
It's worth noting that stereoisomers can be further categorized into configurational stereoisomers and conformational stereoisomers. Configurational stereoisomers have a precise specification of the spatial arrangement of groups, while conformational stereoisomers differ in their conformation.
Executive Orders: Constitutional Power or Overreach?
You may want to see also




























