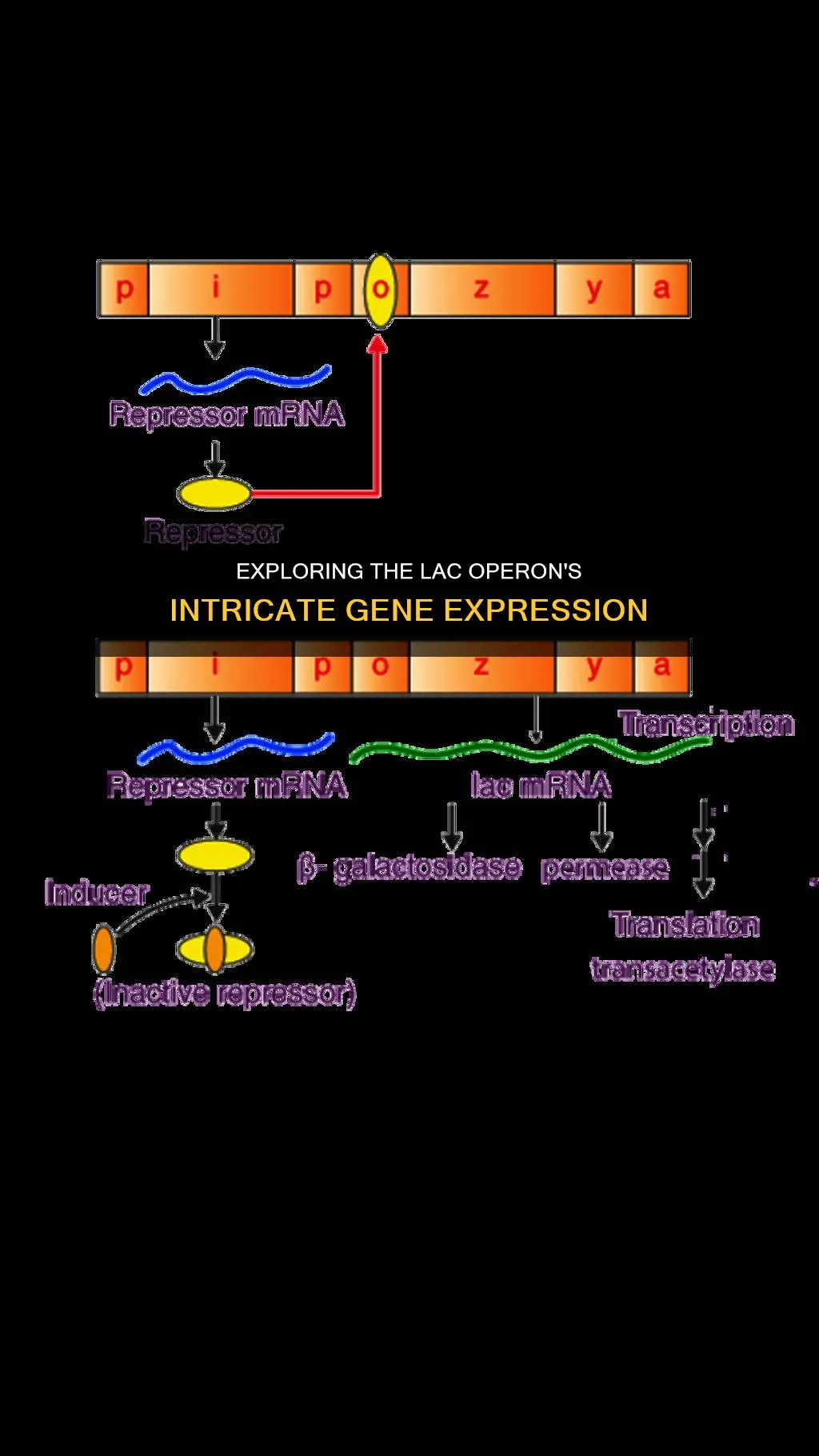
The lac operon is an operon required for the transport and metabolism of lactose in E. coli and many other enteric bacteria. It consists of three structural genes: lacZ, lacY, and lacA. These genes encode for the enzymes beta-galactosidase, beta-galactoside permease, and beta-galactoside transacetylase, respectively. The lac operon is typically induced by the presence of lactose, which acts as an inducer by inactivating the repressor protein, allowing transcription of the operon. Mutations in the operator region can lead to constitutive mRNA synthesis, resulting in the expression of the structural genes even in the absence of lactose. However, the basal level synthesis of lac mRNA ensures a small amount of protein production even without lactose induction. In this context, the discussion revolves around the non-constitutive nature of the lac operon's structural genes and the mechanisms regulating their expression.
Explore related products
What You'll Learn

The lac operon consists of 3 structural genes
The lac operon consists of three structural genes: lacZ, lacY, and lacA. These genes are responsible for the transport and metabolism of lactose in E. coli and other enteric bacteria. The lacZ gene encodes the enzyme β-galactosidase, which cleaves lactose into glucose and galactose. The lacY gene encodes β-galactoside permease, a transmembrane symporter that pumps β-galactosides, including lactose, into the cell. The lacA gene encodes β-galactoside transacetylase, an enzyme that transfers an acetyl group from acetyl-CoA to thiogalactoside.
The lac operon is an important tool for mutant selection and is often used in research. The blue-white screening technique, for example, uses E. coli cells missing a functional lacZ gene and a cloning vector with a modified lacZ gene and an antibiotic resistance gene. This technique allows researchers to easily identify viable colonies for downstream cloning steps.
The lac operon is typically induced by the presence of lactose, which acts as an inducer by inactivating the repressor protein, allowing transcription of the operon. However, the presence of lactose is not sufficient to turn on the expression of the lac operon. The concentration of glucose must also be low or absent. Under these conditions, LacI binds with allolactose, a disaccharide similar to lactose, and the catabolite activator protein (CAP) is expressed, forming a complex with cyclic adenosine monophosphate (cAMP). This double requirement prevents the lac operon from being expressed when an easier-to-metabolize sugar, like glucose, is present, saving energy for cells.
The lac operon also has a positive regulatory control system to prevent wasteful energy generation when excess glucose is present. This system ensures that the cell does not produce enzymes for lactose metabolism when both glucose and lactose are available, as the enzymes for glucose utilization in E. coli are constitutive.
The Constitution: Making the Impossible, Possible
You may want to see also

lacZ, lacY, and lacA are the three structural genes
The lac operon consists of three structural genes: lacZ, lacY, and lacA. These genes are responsible for the transport and metabolism of lactose in E. coli and other enteric bacteria.
The lacZ gene encodes the enzyme β-galactosidase, which is responsible for breaking down lactose into glucose and galactose. This process is essential for bacteria to utilize lactose as an energy source. The lacY gene encodes the protein lactose permease, which is embedded in the bacterial cell membrane. It facilitates the active transport of lactose into the cell, ensuring its availability for β-galactosidase to act upon. The presence of the lacY gene ensures efficient import of lactose, which is crucial for its subsequent breakdown and utilization.
The lacA gene encodes the enzyme thiogalactoside transacetylase, also known as galactoside O-acetyltransferase. While its exact role in lactose metabolism is less clear, it is believed to be involved in detoxification processes related to certain byproducts of lactose metabolism. Thiogalactoside transacetylase may help manage the byproducts or analogs of lactose, preventing their accumulation to toxic levels within the bacterial cell.
The lac operon is a negatively controlled inducible system, with an additional positive regulatory control system. This dual control mechanism prevents wasteful energy generation in the presence of excess glucose. The lac operon is expressed when lactose is present and glucose concentrations are low, allowing the structural genes to be expressed.
US Border: Are Constitutional Rights Upheld?
You may want to see also

lacZ encodes β-galactosidase
The lac operon consists of three structural genes: lacZ, lacY, and lacA. The lacZ gene encodes β-galactosidase (LacZ), an enzyme that plays a crucial role in lactose metabolism by breaking down lactose into glucose and galactose. This process is known as hydrolysis, and it is essential for the effective digestion of lactose in the absence of glucose.
Β-galactosidase is a key enzyme in the lactose operon, which is responsible for the transport and metabolism of lactose in E. coli and other enteric bacteria. The lacZ gene, through its product β-galactosidase, is central to the regulation of gene expression in the lac operon. The presence or absence of lactose in the environment directly impacts the expression of the lacZ gene and the subsequent production of β-galactosidase.
When lactose is present and glucose concentrations are low, the LacI protein binds with allolactose, a lactose metabolite. This binding triggers the expression of the rest of the lac operon, including the lacZ gene. The synthesis of β-galactosidase is directly influenced by the availability of lactose, as it is formed through the reaction of β-galactosidase with lactose. This reaction results in the production of allolactose, which is the natural inducer of the lac operon.
The lacZ gene and its product, β-galactosidase, are not only essential for lactose metabolism but also have significant applications in research and biotechnology. The blue-white screening technique utilises the lacZ gene and β-galactosidase to enable researchers to quickly identify and select bacterial colonies for downstream cloning steps. This technique takes advantage of the ability of β-galactosidase to hydrolyze X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside), producing a blue product that is easily visible to the naked eye.
In summary, the lacZ gene encodes β-galactosidase, an enzyme that is vital for lactose metabolism and gene regulation in the lac operon. The expression of lacZ and the subsequent production of β-galactosidase are directly influenced by the presence of lactose, which initiates a series of reactions leading to the synthesis of allolactose, the natural inducer of the lac operon. Additionally, β-galactosidase plays a central role in research methodologies, such as blue-white screening, making it a well-studied and important enzyme in biology and biotechnology.
Immigration Ban: Unconstitutional?
You may want to see also
Explore related products

lacY encodes β-galactoside permease
The lac operon consists of three structural genes: lacZ, lacY, and lacA. The lac operon is required for the transport and metabolism of lactose in E. coli and other enteric bacteria. The lacZ gene produces the enzyme beta-galactosidase, which breaks down lactose into glucose and galactose.
LacY, the focus of this discussion, encodes β-galactoside permease, a membrane protein that becomes embedded in the plasma membrane. This protein is responsible for the transport of lactose into the cell. It is a transmembrane symporter that pumps β-galactosides, including lactose, into the cell using a proton gradient in the same direction. The permease increases the permeability of the cell to β-galactosides.
The mechanism of β-galactoside permease involves hydronium ions from outside the cell binding to a carboxyl group on the enzyme, allowing it to undergo a conformational change. This conformational change enables the enzyme to bind lactose from outside the cell and transport it inward. The LacY protein has been observed to adopt an outward-facing conformation in its first state, rapidly transitioning to the second state through the binding of a hydrogen ion.
The lacY gene of Escherichia coli has been modified through oligonucleotide-directed, site-specific mutagenesis, resulting in the replacement of histidine residues with arginine residues. The LacY gene is an essential component of the lac operon, encoding lactose permease, a protein that breaks down lactose into glucose and galactose. The absence of lactose permease impairs lactose entry into the cell, hindering subsequent metabolic processes. Thus, lactose permease is crucial for utilising lactose as an energy source.
Enumerated Powers: Congress and the Constitution
You may want to see also

lacA encodes β-galactoside transacetylase
The lac operon consists of three structural genes: lacZ, lacY, and lacA. The lac operon is required for the transport and metabolism of lactose in E. coli and other enteric bacteria. The lacZ gene produces the enzyme beta-galactosidase, which breaks down lactose into glucose and galactose. The lacY gene produces the beta-galactoside permease protein, which is responsible for pumping lactose and other β-galactosides into the cell.
The lacA gene encodes β-galactoside transacetylase, an enzyme that transfers an acetyl group from acetyl-CoA to β-galactosides, glucosides, and lactosides. The biological role of β-galactoside transacetylase is still unclear. However, it may be involved in detoxifying non-metabolizable pyranosides by acetylating them and preventing their re-entry into the cell. The structural and sequence similarities of β-galactoside transacetylase to other members of the acyltransferases superfamily confirm its classification as a CoA-dependent acetyltransferase specific for the 6-hydroxyl group of certain pyranosides.
The lac operon is regulated by the lac repressor, which binds to the lac operator and prevents the initiation of transcription of lac mRNA. When lactose is present and glucose concentrations are low, the LacI protein binds with allolactose, a structural isomer of lactose, and the rest of the operon is expressed. This double requirement ensures that the lac operon is not expressed when glucose, a more easily metabolized sugar, is present, thus saving energy for the cell.
The lac operon has been extensively studied and was the first genetic regulatory mechanism to be clearly understood. Its discovery led to a Nobel Prize in Physiology in 1965 for François Jacob and Jacques Monod. The lac operon is also used as a tool for mutant selection and in the blue-white screening technique, which allows researchers to easily identify viable colonies for downstream cloning steps.
The Constitution's Formative Years: How Did It Begin?
You may want to see also
Frequently asked questions
There are three structural genes of the lac operon: lacZ, lacY, and lacA.
lacZ encodes β-galactosidase (LacZ), an intracellular enzyme that cleaves the disaccharide lactose into glucose and galactose. lacY encodes β-galactoside permease (LacY), a transmembrane symporter that pumps β-galactosides, including lactose, into the cell. lacA encodes β-galactoside transacetylase (LacA), an enzyme that transfers an acetyl group from acetyl-CoA to thiogalactoside.
The lac operon is an operon required for the transport and metabolism of lactose in E. coli and many other enteric bacteria. It is a negatively controlled inducible system that allows for the effective digestion of lactose when glucose is not available.
The lac operon is typically induced by the presence of lactose, which acts as an inducer by inactivating the repressor protein, allowing transcription of the operon.
When lactose is present and glucose concentrations are low, the LacI protein binds with allolactose, a disaccharide structurally similar to lactose. This binding releases LacI from the promoter sequence, allowing the rest of the lac operon to be expressed.






































