Pentane, an organic compound with the formula C5H12, has three structural constitutional isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers, which are molecules with the same molecular formula but different structural formulas, have a range of applications, including as blowing agents in the production of polystyrene foam, as solvents in laboratories, and as components in automobile gasoline. The term isomer originates from the Greek words isos and meros, meaning equal parts.
| Characteristics | Values |
|---|---|
| Number of structural constitutional isomers | 3 |
| Names of isomers | n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane) |
| Molecular formula | C5H12 |
| CAS number of n-pentane | 109-66-0 |
| Synonym of n-pentane | Amyl hydride |
| CAS number of isopentane | 78-78-4 |
| Synonyms of isopentane | 2-Methylbutane, Ethyldimethyl methane |
| CAS number of neopentane | 463-82-1 |
| Synonyms of neopentane | 2,2-Dimethylpropane, tert-Pentane, Tetramethylmethane |
| Boiling points of isomers | Range from about 9 to 36 °C |
| Melting point of isopentane | 30 °C lower than that of n-pentane |
| Melting point of neopentane | 100 °C higher than that of isopentane |
Explore related products
What You'll Learn
- Pentane's structural isomers: n-pentane, isopentane, and neopentane
- Isomers are compounds with the same formula but different structures
- Isopentane is used in high-octane fuels
- Pentanes are used in polystyrene foam production
- N-pentane, isopentane, and neopentane have different boiling and melting points

Pentane's structural isomers: n-pentane, isopentane, and neopentane
Pentane has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers are compounds with the same chemical formula, C5H12, but different chemical structures and properties. The differences in structure lead to variations in boiling and melting points, stability, vapour pressure, and flash points.
N-pentane, also known as linear C5 hydrocarbon, is a straight-chain isomer with the chemical formula C5H12 and a boiling point of 36.1°C. It is the preferred blowing agent for expanded polystyrene and polyethylene manufacturing and is used as a solvent in column chromatography.
Isopentane, also known as 2-methylbutane, is a branched-chain isomer with the same chemical formula, C5H12, as n-pentane. It has a boiling point of 28°C and exists as a colorless, transparent, volatile liquid with a pleasant aroma.
Neopentane, also referred to as 2,2-dimethylpropane or tert-pentane, is another branched-chain isomer of pentane. It has a lower boiling point of 10°C and is a gas at room temperature. Neopentane is toxic and has various applications, including in the manufacture of ice and low-temperature thermometers.
The different structural isomers of pentane exhibit distinct physical and chemical properties, making them suitable for specific applications in industries such as aerospace, automobile, electronics, and defense manufacturing. They are also used in solvent extraction processes, laboratory solvents, and as raw materials for producing industrial chemicals.
Overall, the structural isomers of pentane—n-pentane, isopentane, and neopentane—offer a range of unique characteristics that contribute to their importance in various industrial and scientific contexts.
Fiji's Constitutional Journey Since 1970: A Story of Change
You may want to see also

Isomers are compounds with the same formula but different structures
Pentane (C5H12) is a molecule that exhibits isomerism. It has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers differ in the branching of their carbon backbone, which affects their stability. Generally, more branched isomers are more stable due to their lower surface area and reduced van der Waals forces.
The stability of isomers influences their enthalpy of combustion. Enthalpy of combustion is the heat released when one mole of a substance is completely burned in oxygen. Less stable isomers may release more energy during combustion due to the greater energy difference between reactants and products. Therefore, the two isomers of pentane, n-pentane and isopentane, can be expected to exhibit different enthalpies of combustion due to their structural differences.
The concept of isomers is crucial in understanding the behaviour of molecules. The structural differences between isomers can lead to variations in their physical and chemical properties, impacting their reactivity and energy content. By studying isomerism, scientists can gain insights into the underlying structural arrangements that drive these differences. This knowledge is particularly important in fields such as chemistry and pharmacology, where the unique properties of isomers can have significant implications for reactions and applications.
The American Constitution: Monopolies and Their Impact
You may want to see also

Isopentane is used in high-octane fuels
Pentane has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). Isopentane, in particular, is used in the production of high-octane fuels.
Isopentane, also known as methyl butane, is a structural isomer of pentane with the chemical formula C5H12. It is produced through the acid-catalyzed isomerization of n-pentane. Isopentane has a lower melting point than n-pentane, but its branched isomer, neopentane, has an even lower melting point due to its higher stability.
The use of isopentane in high-octane fuels is of particular interest due to its ability to resist self-ignition. Octane ratings are important in determining the performance and versatility of fuels, especially in aviation gasoline. High-octane fuels allow for a wider range of operating conditions, making them suitable for use in aircraft engines.
The production of high-octane, unleaded motor fuel can be achieved through the alkylation of isobutane with isoamylenes obtained by the dehydrogenation of isopentane. This process involves introducing a mixture of amylenes and unconverted isopentane into an HF alkylation unit for reaction with fresh or recycled isobutane. The alkylation products are then separated into high-octane alkylates, with isopentane being recycled back into the dehydrogenation reactor.
Isopentane's role in the production of high-octane fuels is significant, and its unique chemical properties contribute to its effectiveness in this application. Its low boiling point, low cost, and relative safety further enhance its suitability as a component in the development of high-performance fuels.
Federal Government: Constitutional Congress and the Founding Fathers
You may want to see also
Explore related products
$54.99
$131.25 $175

Pentanes are used in polystyrene foam production
Pentane has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers are compounds with the same chemical formula but different structural arrangements of atoms.
Now, onto the role of pentanes in polystyrene foam production. Polystyrene is a versatile plastic material with a wide range of applications, from food trays and packaging to electronic housings and insulating panels. It is created through the free-radical polymerization of petroleum or its derivative phenylethene (styrene), forming hydrocarbon monomers that create polystyrene through covalent bonds with phenol groups.
Pentanes, specifically n-pentane and isopentane, play a crucial role in the production of polystyrene foam. They are used as blowing agents, which are liquid substances that, when applied, help shape the polystyrene into a harder, foamed material. This foam is often used for insulation due to its sustainability, durability, and improved thermal resistance.
The use of pentanes in polystyrene foam offers several advantages. Firstly, pentanes quickly diffuse out of the material, allowing air to replace them, which contributes to their effectiveness as blowing agents. Secondly, polystyrene itself is chemically non-reactive, making it suitable for a range of industries, including food, medical, biomedical, and pharmaceutical. Additionally, polystyrene is thermoplastic, becoming pliable when heated and rigid when cold, which facilitates easy recycling and remolding.
However, it's important to note that polystyrene is non-biodegradable, emphasizing the importance of diligent recycling to mitigate its environmental impact. In terms of insulation performance, polystyrene foam is less effective than polyurethane foam, which can be made thinner while achieving the same insulating properties. Nevertheless, polystyrene foam remains a popular choice due to its cost-efficiency and the ability to customize its properties by blending different types of pentanes.
Fruity Meals: Lunch or Just a Snack?
You may want to see also

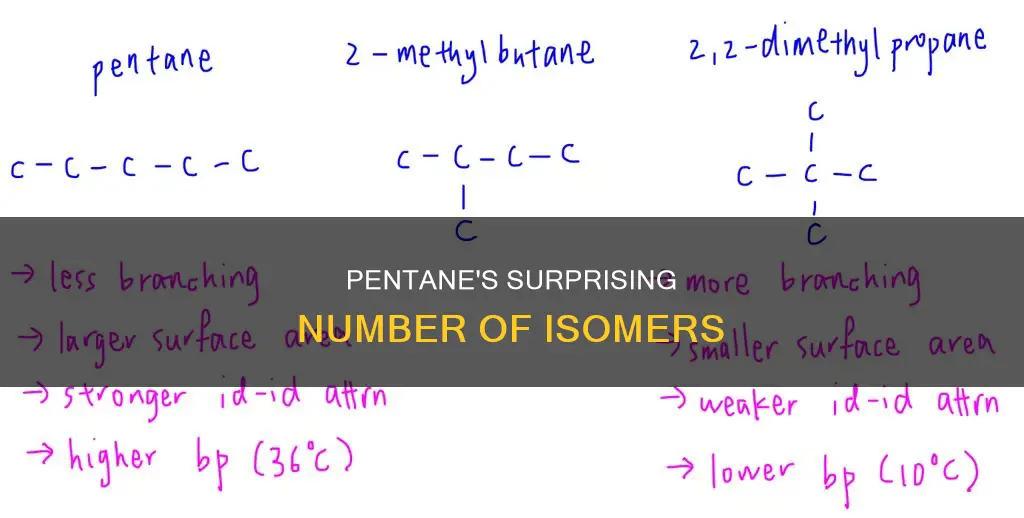
N-pentane, isopentane, and neopentane have different boiling and melting points
Pentane has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). While these compounds share the same chemical formula (C5H12), they differ in their structural arrangements and exhibit distinct physical properties, particularly in terms of their boiling and melting points.
N-pentane, isopentane, and neopentane have varying boiling and melting points due to differences in their molecular shapes and resulting surface areas. N-pentane possesses a greater surface area compared to neopentane. The larger surface area of n-pentane results in stronger intermolecular attractive forces, leading to a higher boiling point of 36.1°C, while neopentane, with a lower surface area, has a boiling point of 10°C.
The molecular shape of neopentane is spheroid, which facilitates efficient packing into a solid structure. In contrast, n-pentane has more possible conformations, allowing it to remain in a liquid state over a broader temperature range. Consequently, n-pentane exhibits a lower melting point compared to neopentane.
The difference in behaviour between n-pentane and neopentane can be attributed to their distinct shapes. The greater surface area of n-pentane results in stronger intermolecular forces, favouring the liquid state. Conversely, the spheroid shape of neopentane facilitates efficient packing in the solid state, making it more inclined to exist in the solid state over the liquid state.
In summary, the differences in boiling and melting points among n-pentane, isopentane, and neopentane arise from variations in their molecular shapes and resulting surface areas. These shape and surface area differences influence the intermolecular forces and, consequently, the physical states and phase transition temperatures of these isomers.
Bernie Sanders' Speech: Echoes of Stalin's Constitution?
You may want to see also
Frequently asked questions
Pentane has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane).
Isomers are molecules with the same molecular formula but different structural formulas.
The molecular formula of pentane is C5H12.
Pentanes are used as blowing agents in the production of polystyrene foam and other foams. They are also used in some fuels, refrigerants, and pesticides.











































