The molecular formula C6H14 represents an alkane, a type of hydrocarbon that only contains single bonds. Constitutional isomers are compounds that share the same molecular formula but differ in the connectivity of their atoms. For C6H14, there are five constitutional isomers, including hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane. These isomers represent different structural arrangements of the six-carbon alkane without rings or multiple bonds.
| Characteristics | Values |
|---|---|
| Number of possible constitutional isomers of C6H14 | 5 |
| Names of the isomers | 2-Methylpentane, 3-Methylpentane, 2,2-Dimethylbutane, 2,3-Dimethylbutane, and 3,3-Dimethylbutane |
| Molecular formula | C6H14 |
| Molecular weight | 86.18 g/mol |
| Boiling Point | Varies with the isomer, but ranges from -20 to 60 degrees Celsius |
| Melting Point | Varies with the isomer, but ranges from -160 to -120 degrees Celsius |
| Density | Approximately 0.68 g/cm3 at 20 degrees Celsius (varies with the isomer) |
| Solubility | Slightly soluble in water, highly soluble in organic solvents |
| Flash Point | Varies, but generally below room temperature |
| Autoignition Temperature | Not readily available, but expected to be higher than the boiling point |
| Odor | Characteristic hydrocarbon odor |
| Health Effects | May cause eye, skin, and respiratory irritation. Inhalation of vapors may cause dizziness and headache. |
Explore related products
What You'll Learn
- There are five constitutional isomers of C6H14
- These include hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane
- They are all structural isomers of the six-carbon alkane
- Each isomer has the same molecular formula but differs in the arrangement of carbon atoms
- This is known as chain isomerism

There are five constitutional isomers of C6H14
The five isomers of C6H14 are:
- Hexane: a straight-chain alkane with all six carbon atoms connected in a line.
- 2-Methylpentane: a branched-chain alkane where one methyl group (CH3) is attached to the second carbon of a five-carbon chain.
- 3-Methylpentane: another branched-chain isomer with a methyl group attached to the third carbon of a five-carbon chain.
- 2,2-Dimethylbutane: this isomer has two methyl groups attached to the second carbon of a four-carbon chain. It is also known as neohexane.
- 2,3-Dimethylbutane: this isomer has two methyl groups, differentiating it from 2,2-dimethylbutane.
These five structural arrangements of C6H14 provide different properties for each compound, despite their identical molecular formulas.
Authentic Constitution Copies: What's the Real Deal?
You may want to see also

These include hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane
There are five possible constitutional isomers of the chemical compound C6H14: hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane. These isomers are organic compounds known as alkanes, which are molecules composed of carbon and hydrogen atoms. Alkanes are further classified based on the number of carbon atoms in their chemical structure, with the prefix indicating the number of carbon atoms and the suffix "-ane" denoting a single bond between carbon atoms. In the context of C6H14, the prefix "hex-" indicates six carbon atoms.
Hexane, also known as n-hexane, is a straight-chain alkane with six carbon atoms arranged in a linear structure. It has the molecular formula C6H14 and is a colorless, odorless liquid with a boiling point of approximately 69 °C (156 °F). Hexane is commonly obtained through the refinement of crude oil, and it finds wide-ranging applications across industries. For instance, it serves as a non-polar solvent in laboratory settings for extracting oil and grease contaminants from water and soil. Additionally, hexane is utilized in the formulation of adhesives for shoes, leather goods, and roofing materials. Modern gasoline blends also typically contain about 3% hexane.
Moving on to the other isomers, 2-methylpentane and 3-methylpentane are structural isomers of hexane, featuring the same molecular formula, C6H14, but differing in the arrangement of their carbon and hydrogen atoms. These isomers are often found in mixtures referred to as "hexanes," which are cheaper than pure hexane and find utility in cleaning solvents and chromatography. 2,2-dimethylbutane and 2,3-dimethylbutane, on the other hand, are isomers with the molecular formula C6H14 but differ in the positioning of their carbon and hydrogen atoms.
While all these isomers share the same molecular formula, C6H14, they exhibit distinct physical and chemical properties due to differences in their structural arrangements. The variation in the arrangement of atoms leads to changes in their boiling points, melting points, solubility, and reactivity. These unique characteristics of each isomer contribute to their specific applications and behaviors in chemical processes.
The House: Constitution's Membership Rules
You may want to see also

They are all structural isomers of the six-carbon alkane
Alkanes are a family of molecules that are hydrocarbons, meaning they contain only carbon and hydrogen. They are often described as saturated hydrocarbons because they have only carbon-carbon and carbon-hydrogen single bonds, and they contain the maximum possible number of hydrogens per carbon. The general formula for alkanes is CnH2n+2, where n is an integer. The first six alkanes are:
- Methane (CH4)
- Ethane (C2H6)
- Propane (C3H8)
- Butane (C4H10)
- Pentane (C5H12)
- Hexane (C6H14)
Alkanes with four or more carbon atoms show structural isomerism, meaning that there are two or more different structural formulas for each molecular formula. For example, C4H10 can be either butane or 2-methylpropane (isobutane). Similarly, C5H12 has three possible isomers: pentane, isopentane, and neopentane. These compounds are all isomers of the five-carbon alkane, and they have the same molecular formula but different structures and properties, including different boiling points.
C6H14, or hexane, is the straight-chain isomer of the six-carbon alkane. It has a continuous chain of six carbon atoms and is represented by the condensed structure CH3(CH2)4CH3. There are a total of five possible structural isomers of C6H14, including the straight-chain isomer. The other four isomers have branching at the second and third carbon atoms, resulting in different structures and properties compared to the straight-chain isomer.
The identification and naming of isomers follow specific rules. The first step is to find and number the longest continuous carbon chain, which becomes the parent name. The second step is to number the parent chain, starting from the end closest to the first substituent. The third step is to identify and name any groups attached to the parent chain, and the fourth step is to put these substituent groups in alphabetical order. Finally, the substituents are located on the parent chain by giving them numbers.
Understanding the Core Duties of US Congress
You may want to see also
Explore related products

Each isomer has the same molecular formula but differs in the arrangement of carbon atoms
Isomers are molecules or polyatomic ions with identical molecular formulas, meaning they have the same number of atoms of each element. However, isomers differ in the arrangement of their atoms in space. This concept is known as isomerism, which refers to the existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties, and their distinct structural arrangements can lead to differences in chemical reactivity and physical properties such as boiling and melting points.
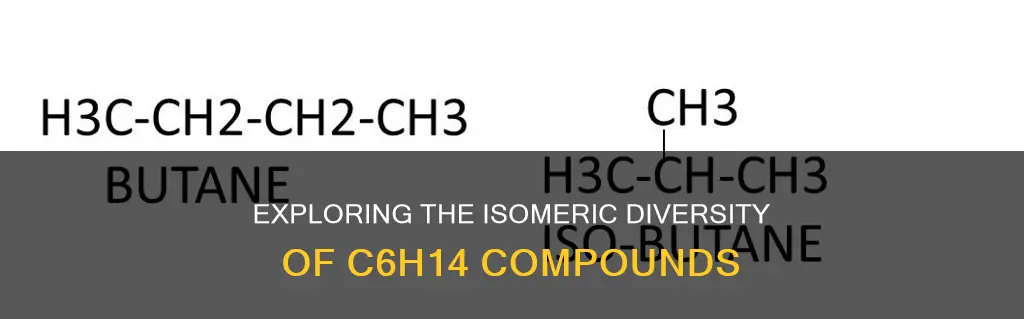
There are two main forms of isomerism: structural (or constitutional) isomerism and stereoisomerism (or spatial isomerism). In structural isomerism, the bonds between the atoms differ, while in stereoisomerism, the bonds are the same, but the relative positions of the atoms differ. For example, butane and isobutane are structural isomers with the formula C4H10, but they differ in the arrangement of their carbon atoms, resulting in different physical properties.
Another type of isomerism is geometric isomerism, also known as cis-trans isomerism, which occurs in compounds with restricted rotation, such as double bonds or ring structures. Geometric isomers can occupy different positions relative to each other. Optical isomers, also known as enantiomers, are a type of stereoisomer that are mirror images of each other, like left and right hands. They can rotate plane-polarized light in different directions. Stereoisomers can also arise from tetrahedral carbon atoms attached to four different substituents, resulting in non-superimposable mirror image molecules.
Spin isomerism is a type of isomerism based on nuclear properties, where molecules differ in the relative spin magnetic quantum numbers of their constituent atomic nuclei. This is significant for molecular hydrogen, which can exist as parahydrogen and orthohydrogen, with nuclear spins pointing in opposite and the same directions, respectively. The placement of functional groups, such as methyl groups, can also lead to isomerism, resulting in distinct biological properties.
Founding Fathers: Biblical Principles in the Constitution
You may want to see also

This is known as chain isomerism
Isomerism is a phenomenon where two or more compounds share the same chemical formula but have different structural formulae and characteristics. This phenomenon is observed due to differences in the structural and spatial arrangements of atoms. When two or more compounds have the same molecular formula but distinct structural formulas, it is referred to as structural isomerism, which includes chain isomerism.
Chain isomerism occurs when the carbon skeleton of the compounds is arranged differently, leading to various linear and branched forms. This phenomenon is observed in alkanes, which are hydrocarbons with single bonds between carbon atoms. Alkanes with four or more carbon atoms can form chain isomers. For example, n-butane and isobutane are two structural isomers with the molecular formula C4H10 but differ in the arrangement of carbon atoms, making them chain isomers.
The molecular formula C4H10 indicates an alkane with four carbon atoms and ten hydrogen atoms but does not reveal the structure of the molecule. This is where isomerism becomes important, as it allows us to predict and draw different isomeric structures. Understanding isomerism is crucial for comprehending the diverse behaviour of organic molecules in chemical reactions and their applications in various fields of science and industry.
In the petrochemical industry, chain isomerization is employed to produce more branched alkanes with higher octane numbers from linear alkanes, resulting in fuels better suited for petrol engines. Straight-chain alkanes are heated with catalysts to break up the chains, leading to the formation of more branched alkanes and lower alkanes as the pieces recombine. The octane number of an alkane indicates its level of branching, with higher numbers indicating greater branching and reduced auto-ignition propensity in automobile engines.
Constitutional Amendments of 1945: What Changed?
You may want to see also
Frequently asked questions
There are five constitutional isomers possible for the formula C6H14. These include hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane.
Constitutional isomers are compounds that have the same molecular formula but differ in the connectivity of their atoms.
The isomers of C6H14 are called chain isomers because they have the same molecular formula but differ in the arrangement of carbon atoms in their chains.