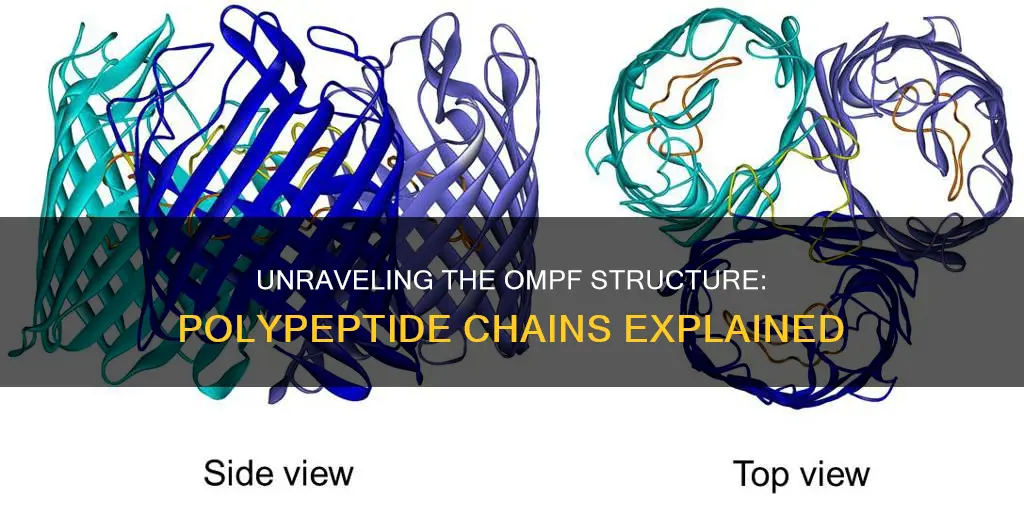
Proteins are structurally complex and functionally sophisticated molecules. The structure and chemistry of each protein have been developed and fine-tuned over billions of years of evolutionary history. The structure of a protein is determined by its amino acid sequence. The primary structure of a protein is defined as the sequence of amino acids linked together to form a polypeptide chain. The secondary structure is the local spatial arrangement of a polypeptide's backbone (main chain) atoms, and the tertiary structure refers to the three-dimensional structure of an entire polypeptide chain. When a protein is formed as a complex of more than one polypeptide chain, it is referred to as having a quaternary structure. This added complexity requires an assembly process of the separate polypeptide chains to create a functional unit. In this context, we will explore the functional unit of OmpF, an outer membrane porin protein.
Explore related products
What You'll Learn

OmpF is a porin, a non-specific pore
The OmpF porin is composed of a 16-stranded β-barrel that contains the channel. Six loops are exposed at the surface of the cell, and one of these loops, L3, plays a crucial role in constricting the pore. The loop L3 is bent into the channel, narrowing the pore and regulating the passage of molecules. Mutations in the loop L3 region can alter the pore size and affect the selectivity of the OmpF porin. For example, mutations that substitute shorter amino acid residues for longer ones in the loop L3 region can lead to a larger pore size, allowing the passage of larger molecules.
The OmpF porin also exhibits an electrostatic field established by the interaction of positively and negatively charged residues within the pore. This electrostatic field influences the movement of charged molecules through the pore. Mutations that affect the charged residues can modify the electrostatic field and, consequently, the selectivity of the pore. Additionally, the OmpF porin is known to undergo conformational changes upon interacting with other molecules in the cell, which can further modulate its pore functionality.
The non-specific nature of the OmpF porin allows it to facilitate the transport of a diverse range of molecules. It plays a vital role in the selective uptake of essential nutrients and the secretion of metabolic waste products. Moreover, the OmpF porin is involved in the transport of antibiotics, and its structure and function are crucial in the development of bacterial resistance. Understanding the structure-function relationship of the OmpF porin is essential for designing strategies to combat bacterial resistance and improve the effectiveness of antibiotic treatments.
In summary, OmpF is a porin that forms a non-specific pore in the outer membrane of Escherichia coli. Its structure, including the β-barrel, loops, and electrostatic field, contributes to its functionality as a non-specific pore, allowing the passage of various molecules. Mutations in the OmpF porin can alter its pore size and selectivity, highlighting the dynamic nature of this protein. The study of OmpF and its role in membrane transport has significant implications for understanding bacterial resistance and developing effective therapeutic approaches.
Company Car Commercial Use: What You Need to Know
You may want to see also

OmpF is involved in drug transport
OmpF is a porin, or channel, in the outer membrane of Gram-negative bacteria. It is involved in the transport of antibiotics and other drugs across the membrane and into the cell. Understanding how this process works is important for developing new antibiotics that can effectively fight against antibiotic resistance in bacteria.
The outer membrane of Gram-negative bacteria is the first permeability barrier that protects the cells from environmental stresses, including chemical, biophysical, and biological attacks. At the same time, it allows the selective uptake of essential nutrients and the secretion of metabolic waste products. This membrane controls the drug concentration in the bacterial cell, contributing to antibiotic susceptibility.
OmpF is one of the porins that allow antibiotics to traverse the outer membrane of Gram-negative bacteria. These porins have the ability to transition between open and closed states, regulating transport properties and rates. The opening and closing of the OmpF porin are thought to be controlled by the motion of the internal loop, L3, which leads to widening and narrowing of the pore.
Studies have shown that mutations in the porins of resistant clinical isolates target amino acids of loop L3. For example, the homologous mutation G119D in E. coli OmpF reduces the size of the channel and confers a drastic reduction in β-lactam susceptibility. Understanding the molecular bases of the antibiotic influx mechanism is crucial for developing new antibiotics that can effectively combat bacterial antibiotic resistance.
The transport of drugs across the membrane can occur through passive or active transport, represented by porins and efflux pumps, respectively. The expression of these transporters and channels is regulated by a complex network of systems, including Helix-turn-Helix (HTH) family regulators and two-component systems (TCS). These systems play a key role in bacterial adaptation to environmental stresses and can modulate the expression of transporters to increase antibiotic resistance.
Samuel Adams' Vision for the US Constitution
You may want to see also

OmpF is an outer membrane protein
OmpF is an outer membrane porin protein found in Gram-negative bacteria, such as E. coli and Salmonella enterica. It is involved in a wide range of virulence- and pathogenesis-related cellular processes, including transport, adhesion, penetration, and the colonisation of host tissues.
The outer membrane of Gram-negative bacteria acts as a protective barrier against environmental stresses, while also facilitating the selective uptake of essential nutrients and the secretion of metabolic waste products. This membrane has a complex asymmetrical architecture, with phospholipids in the inner leaflet and lipopolysaccharide (LPS) molecules in the outer leaflet.
OmpF is one of the outer membrane proteins found in this environment, alongside OmpA, FhuA, EstA, BtuB, and OmpX. Each of these proteins has a unique pattern of interaction with the surrounding membrane, influenced by its composition, the level of LPS in the outer leaflet, and the differing mobilities of the lipids in the two leaflets.
OmpF has a specific LPS-binding region on its outer surface, and it interacts with other proteins such as BtuB to facilitate the translocation of vitamin B12 across the outer membrane.
In terms of structure, OmpF shares the typical β-barrel spatial structure of most outer membrane porins, which provides structural integrity within the membrane lipid bilayer. However, recent data suggest that OmpF can also adopt an amyloid state, forming amyloid fibrils at the surface of E. coli cells.
In terms of drug resistance, OmpF plays a role in the transport of antibiotics through the OmpF/C general porins and TolC-like channels. Mutations in the porins of resistant clinical isolates can lead to modifications in membrane permeability and reduced susceptibility to antibiotics.
Who Were the Slave-Owning Framers of the Constitution?
You may want to see also
Explore related products

OmpF is involved in bacterial resistance
OmpF is a porin, or outer membrane protein, that is found in the outer membrane (OM) of Gram-negative bacteria. The OM acts as a barrier, protecting the bacteria against environmental stresses and toxic chemicals, including antibiotics.
OmpF is involved in the development of bacterial resistance to antibiotics. This is due to its role in the passive transport of various molecules, including antibiotics, across the OM. OmpF is a non-specific porin, which means it allows the passage of a range of different molecules. This includes β-lactams, fluoroquinolones, and other toxic agents.
The role of OmpF in antibiotic transport has been demonstrated in several studies. For example, an OmpF-defective mutant of Escherichia coli was found to be resistant to several antibiotics, including β-lactams. This suggests that OmpF is a key route for the penetration of antibiotics into the bacteria.
The expression of the OmpF gene can be affected by various factors, including high osmolarity conditions and the efflux pump gene AcrB, which has been shown to repress the expression of OmpF. Additionally, point mutations can affect the synthesis of OmpF, leading to diminished or absent synthesis of the protein in the OM. This altered expression of OmpF can contribute to bacterial resistance to antibiotics, specifically carbapenem resistance.
The balance between OmpF and another porin, OmpC, also plays a role in bacterial resistance. The downregulation of OmpF, along with increased expression of OmpC, has been associated with carbapenem resistance in Enterobacter isolates. Overall, the involvement of OmpF in bacterial resistance is complex and influenced by various factors, including gene expression, protein synthesis, and the interaction with other porins.
US Constitution: A Handover or a Hand-off?
You may want to see also

OmpF is a stable trimer
The outer membrane of Gram-negative bacteria plays a crucial role in their resistance to bile, which requires the expression and function of multiple OMPs (outer membrane proteins). OmpF is one such OMP that has been the subject of extensive studies in recent years.
OmpF, or Outer Membrane Protein F, is a porin that contributes to the membrane permeability of Gram-negative bacteria. Porins are the most abundant OMPs and play a key role in the bacterial outer membrane as stable trimers, which are highly resistant to bile salts. The stability of OmpF as a trimer is essential for its function in regulating membrane permeability and protecting the bacteria against environmental stresses.
The structure of OmpF consists of a periplasmic α-helical barrel formed by 12 coiled-coil α-helices, with four contributed by each protomer. This unique structure allows for the passage of large and partially folded proteins. OmpF's channel is not occluded by an inside loop or a plug domain, which is a distinctive feature when compared to other channels like those found in secretins or siderophore transporters.
The stability of the OmpF trimer is further highlighted by its ability to withstand mutations. Studies have shown that mutations in porins of resistant clinical isolates specifically target amino acids in loop L3. For example, the homologous mutation G119D in E. coli OmpF narrows the channel size due to the protrusion of the large side chain of Asp into the channel lumen. Despite this mutation, OmpF retains its functionality, exhibiting a significant reduction in β-lactam susceptibility.
In summary, OmpF is a stable trimer that plays a vital role in the outer membrane of Gram-negative bacteria. Its unique structure and stability contribute to its function in regulating membrane permeability and protecting bacteria from environmental stressors. The stability of the OmpF trimer is evident in its resistance to bile salts and its ability to maintain functionality despite mutations.
Foreign Policy Powers: The US Constitution's Divide
You may want to see also