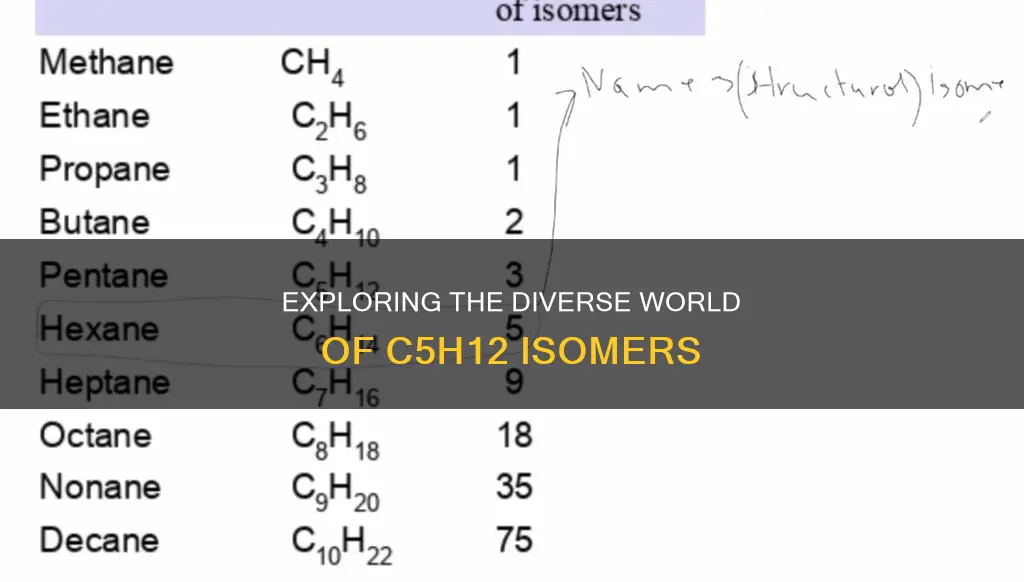
The molecular formula C5H12 refers to isomers of carbon chain isomers, structural isomers, and alkanes. These isomers include pentane, methylbutane, and dimethylpropane. The number of structural isomers of C5H12 is dependent on how the carbon atoms are connected and how many hydrogen atoms are attached to each carbon atom.
| Characteristics | Values |
|---|---|
| Number of different molecules | 2 |
| Names of the molecules | n-pentane and 2-methylbutane |
Explore related products
What You'll Learn

C5H12 has multiple structural isomers
C5H12, also known as pentane, has three structural isomers. The first two isomers are pentane and 2-methylbutane. The third isomer is 2,2-dimethylpropane.
To understand why C5H12 has multiple structural isomers, we need to know what an isomer is. In organic chemistry, an isomer is defined by IUPAC as one of several species (molecular entities) with the same atomic composition (molecular formula) but different two-dimensional representations. In other words, isomers have the same types and numbers of atoms but differ in how those atoms are connected and arranged in space.
In the case of C5H12, the three isomers arise from different arrangements of carbon and hydrogen atoms. Pentane (n-pentane) has a straight chain of five carbon atoms, with hydrogen atoms bonded to each carbon. 2-methylbutane, on the other hand, has four carbon atoms in a straight chain with one methyl group (CH3) attached to the second carbon atom. This creates a branch in the carbon chain, giving it a different structure from pentane.
The third isomer, 2,2-dimethylpropane, also known as neopentane, has an even more branched structure. It consists of three carbon atoms bonded together in a chain, with two methyl groups (CH3) attached to the central carbon atom. This results in a molecule that looks like a tripod, with the methyl groups forming the legs.
These different arrangements of atoms lead to unique molecular structures for each isomer, despite them all sharing the same molecular formula of C5H12. This phenomenon of multiple structural isomers arises due to the flexibility and variability in how carbon atoms can bond with each other and with hydrogen atoms, allowing for the creation of distinct molecular entities with distinct properties.
Exploring Constitution-Class Vessels: Decks and Their Functions
You may want to see also

These include n-pentane and 2-methylbutane
The molecular formula C5H12 represents three structural isomers: n-pentane, isopentane (methylbutane or 2-methylbutane), and neopentane (dimethylpropane). These isomers differ in their molecular structure, physical properties, and applications.
N-pentane, also known as normal pentane, is the unbranched isomer with a straight carbon chain. It has a higher melting point compared to isopentane, but a lower melting point than neopentane, which is the most heavily branched isomer. N-pentane has a lower octane rating than isopentane, making it less desirable for high-octane fuels. However, it finds applications in various other areas, such as a working medium in geothermal power stations and organic Rankine cycles, as well as a solvent in laboratories and certain pesticides.
Isopentane, on the other hand, is a branched-chain saturated hydrocarbon. It is commonly referred to as methylbutane or 2-methylbutane, with the numerical prefix indicating the position of the methyl group on the carbon chain. Isopentane has a lower melting point than n-pentane, and it is highly volatile and flammable. Isopentane is often used in conjunction with liquid nitrogen to achieve extremely low temperatures for applications like tissue freezing in histology. It is also a significant component of natural gasoline, with its high octane rating making it desirable for high-performance fuels.
Neopentane, or dimethylpropane, is the most heavily branched isomer of C5H12. It has the highest melting point among the three isomers due to the tetrahedral arrangement of its molecules in solid form. Neopentane is less dense than the other two isomers and exhibits a significantly lower entropy of fusion. While it is not commonly used in gasoline, it may have specific applications in specialty solvents and other chemical processes.
In summary, n-pentane, 2-methylbutane (isopentane), and neopentane are the three structural isomers of C5H12, each with distinct structural, physical, and chemical characteristics. These isomers have various applications in fuels, refrigeration, laboratory solvents, and other industrial processes, showcasing the importance of understanding their unique properties.
Document Size: Does It Alter the Meaning?
You may want to see also

Single bonds allow free atom rotation about the axis
The molecular formula C5H12 has three isomers: n-pentane, isopentane (also known as methylbutane), and neopentane (also known as dimethylpropane). This response will focus on the fact that single bonds allow free atom rotation about the axis in the context of these molecules.
Single bonds between atoms allow for free rotation around the internuclear axis due to their direct orbital overlap and the symmetrical distribution of electron density along the axis. This symmetrical electron density, characteristic of sigma (σ) bonds, enables the bonded atoms to rotate freely without breaking the bond. It is worth noting that sigma bonds are formed by the direct overlap of two atomic orbitals, typically s-orbitals, p-orbitals, or a combination of the two. These orbitals house two electrons, with each atom contributing one.
In contrast, double and triple bonds restrict rotation due to the presence of pi (π) bonds, which introduce complexity to chemical structures. Pi bonds are formed by the lateral or side-by-side overlap of p-orbitals, resulting in electron densities above and below the internuclear axis. This uneven distribution of electron density creates a region that impedes the free rotation of atoms around each other. Additionally, the closer atomic proximity within multiple bonds increases steric hindrance, further hindering rotation.
The ability of single bonds to rotate freely has important implications for understanding molecular geometry and isomerism. In the case of C5H12 isomers, the free rotation of single bonds allows for different conformations of the molecule. For example, isopentane (2-methylbutane) can exist in multiple conformations due to the rotation of the methyl group around the carbon-carbon single bond. These conformations include the staggered conformation, the eclipsed conformation, and various degrees of rotation between these extremes.
While single bonds allow for free rotation, it is important to consider the impact of this rotation on the energy state of the molecule. Some conformations resulting from bond rotation may have slightly higher energy content due to repulsion between atoms or between groups bonded to adjacent carbons as they approach each other. However, the bond strength remains constant throughout the rotation, and the molecule can exist in an infinite number of stable conformations.
The Complexities of Dichotomous Thinking
You may want to see also
Explore related products

C5H12 has no functional group isomers
C5H12, also known as pentane, has three structural isomers: n-pentane, 2-methylbutane, and 2,2-dimethylpropane. These isomers differ in the way their carbon atoms are connected, resulting in distinct molecular structures. However, C5H12 has no functional group isomers.
To understand why C5H12 has no functional group isomers, it is essential to comprehend the concept of functional groups and their role in isomerism. Functional groups are specific groupings of atoms within a molecule that exhibit characteristic chemical behaviour and play a crucial role in defining the molecule's properties. Isomers, on the other hand, are compounds that have the same molecular formula but differ in the way their atoms are connected, leading to distinct structural arrangements.
In the case of C5H12, the molecular formula indicates that it contains five carbon (C) atoms and twelve hydrogen (H) atoms. The absence of any other elements, such as oxygen or nitrogen, means that there are no alternative arrangements of atoms that can form different functional groups. All the isomers of C5H12 have the same functional group, which is the carbon-hydrogen (C-H) bond.
While C5H12 lacks functional group isomers, it exhibits structural isomerism. Structural isomers, as mentioned earlier, share the same molecular formula but differ in the arrangement of their atoms or the connectivity of their bonds. In the case of C5H12, n-pentane, 2-methylbutane, and 2,2-dimethylpropane are structural isomers that vary in the way carbon atoms are bonded to each other and to hydrogen atoms.
The distinction between functional group isomers and structural isomers is important. Functional group isomers arise when the same functional groups are present but differ in their arrangement or connectivity within the molecule. On the other hand, structural isomers, like those of C5H12, have identical functional groups but differ in the overall structure of the molecule due to differences in bond connectivity.
In summary, C5H12, or pentane, has no functional group isomers because it contains only carbon and hydrogen atoms, resulting in a single type of functional group (C-H bond) across all its isomers. However, it exhibits structural isomerism, with n-pentane, 2-methylbutane, and 2,2-dimethylpropane being the three structural isomers that differ in the arrangement of their carbon and hydrogen atoms.
Local Police and Their Oath to the Constitution
You may want to see also

Nor does it have geometrical or optical isomers
The molecular formula C5H12 does not have geometrical or optical isomers. Isomers are molecules that share the same molecular formula but differ in the arrangement of atoms in space. Geometrical isomers, also known as cis-trans isomers, occur when there is restricted rotation in the molecule, usually involving a carbon-carbon double bond. However, the molecules with the formula C5H12 have carbon-carbon single bonds, allowing free rotation and preventing geometrical isomerism.
Geometrical isomers are only possible with square planar and octahedral structures. They cannot exist in linear or tetrahedral structures. In the case of C5H12, the molecule has a linear structure, making it incapable of forming geometrical isomers.
Optical isomers, also known as stereoisomers, occur when a molecule has a chiral center, meaning it lacks a plane of symmetry. Chiral molecules have four different groups attached to a central carbon atom, resulting in a non-superimposable mirror image. However, C5H12 molecules do not possess a chiral center and exhibit symmetry, making them achiral and incapable of forming optical isomers.
It's important to distinguish between geometrical and optical isomerism. While both types of isomerism involve differences in spatial arrangements, optical isomers specifically refer to isomers that affect plane-polarized light. The absence of a chiral center in C5H12 molecules means they do not exhibit optical isomerism and have no effect on plane-polarized light.
In summary, the formula C5H12 does not lend itself to geometrical or optical isomerism due to its linear structure and the presence of carbon-carbon single bonds that allow free rotation. The symmetry of the molecule further reinforces its inability to form optical isomers, as only chiral molecules with four distinct groups attached to a central carbon atom can exhibit optical isomerism.
US Constitution: Rights for All Residents?
You may want to see also
Frequently asked questions
An isomer is one of several species (molecular entities) that have the same atomic composition (molecular formula) but different two-dimensional representations.
There are two isomers of C5H12: n-pentane and 2-methylbutane.
The molecular formula of C5H12 isomers is C5H12, with a molecular mass of 72.
The structural formulas of C5H12 isomers depend on the specific isomer. For example, n-pentane has the formula H | H — C — H | H H H | | | | H — C — C — C — C — H | | | | H H H H, while 2-methylbutane has a slightly different structure.































