NF-κB is a regulator of genes that control cell proliferation and cell survival. It is widely used by eukaryotic cells and is crucial for the survival of activated B cells. The constitutive activation of NF-κB has been observed in several lymphoid malignancies, including diffuse large B-cell lymphoma, Hodgkin lymphoma, and adult T-cell leukemia. This activation can be caused by various factors, such as persistent infections, a pro-inflammatory microenvironment, self-reactive immune receptors, and genetic lesions. The API2-MALT1 fusion oncoprotein, for instance, induces proteolytic cleavage of NF-κB-inducing kinase (NIK), resulting in deregulated NIK activity and constitutive noncanonical NF-κB signaling. Understanding the mechanisms behind aberrant NF-κB activation is essential for developing targeted antitumor therapies and improving lymphoma treatment.
| Characteristics | Values |
|---|---|
| Role of NF-κB | Regulator of genes that control cell proliferation and cell survival |
| Cause of aberrant NF-κB activation | Persistent infections, pro-inflammatory microenvironment of the cancer, self-reactive immune receptors, genetic lesions |
| Types of cancers with aberrant NF-κB activation | Diffuse large B-cell lymphoma, Hodgkin lymphoma, adult T-cell leukemia |
| Genetic mutations leading to constitutive activation | TNFAIP3 encoding A20, MYD88, CD79A/B |
| Role of RelB-regulated genes | Contribute to the survival and activation of B cells, which are chronically activated by CD40 signaling |
| Role of non-canonical NF-κB pathway | Expansion and lymphomagenesis of CD40-activated B cells |
| Role of BCR signaling | Important in B-cell malignancies |
| Role of TLR-derived signals | Importance in lymphomagenesis is under debate |
Explore related products
What You'll Learn

The role of NF-κB in tumor-precursor cells
NF-κB is widely used by eukaryotic cells as a regulator of genes that control cell proliferation and cell survival. Active NF-κB turns on the expression of genes that keep the cell proliferating and protect the cell from conditions that would otherwise cause cell death via apoptosis. In cancer, proteins that control NF-κB signalling are mutated or aberrantly expressed, leading to defective coordination between the malignant cell and the rest of the organism. This is evident in metastasis, as well as in the inefficient eradication of the tumour by the immune system.
The NF-κB transcription factor family plays a crucial role in lymphocyte proliferation and survival. Consequently, aberrant NF-κB activation has been described in a variety of lymphoid malignancies, including diffuse large B-cell lymphoma, Hodgkin lymphoma, and adult T-cell leukemia. Several factors, such as persistent infections, the pro-inflammatory microenvironment of the cancer, self-reactive immune receptors, and genetic lesions altering the function of key signalling effectors, contribute to constitutive NF-κB activity in these malignancies.
The role of NF-κB in tumour-precursor cells is an area of ongoing research. B-cell tumours originating from the transformation of germinal centre B cells frequently harbour genetic mutations, leading to constitutive activation of the NF-κB signalling pathway. Understanding how aberrant NF-κB activation promotes tumorigenesis requires an understanding of the role of NF-κB in these tumour-precursor cells. Despite extensive knowledge of NF-κB biology, the function of this complex signalling pathway in the differentiation of germinal centre B cells is largely unknown.
The identification of the biological roles of the separate NF-κB transcription factor subunits in germinal centre biology will help to better understand the pathogenic consequences of their constitutive activation in B-cell tumours. This knowledge may be exploited for the development of targeted antitumor therapies aimed at selectively inhibiting those components of aberrant NF-κB activity that contribute to pathogenesis.
Chile's Constitution: Approved or Rejected?
You may want to see also

NF-κB activation and B-cell tumors
NF-κB is a regulator of genes that control cell proliferation and cell survival. It is used by eukaryotic cells and is involved in the development of B-cells. NF-κB activation is required for the survival of activated B-cell-like diffuse large B-cell lymphoma cells.
The NF-κB transcription factor family plays a crucial role in lymphocyte proliferation and survival. As a result, aberrant NF-κB activation has been observed in several lymphoid malignancies, including diffuse large B-cell lymphoma, Hodgkin lymphoma, and adult T-cell leukemia. Constitutive NF-κB activity in these malignancies can be caused by various factors, such as persistent infections, a pro-inflammatory microenvironment, self-reactive immune receptors, and genetic lesions affecting key signalling effectors.
B-cell tumours originating from the transformation of germinal centre B cells frequently harbour genetic mutations, leading to constitutive activation of the NF-κB signalling pathway. Understanding the role of NF-κB in these tumour-precursor cells is crucial for comprehending how aberrant NF-κB activation promotes tumour growth. Identifying the biological roles of the separate NF-κB transcription factor subunits in germinal centre biology will aid in understanding the pathogenic consequences of their constitutive activation in B-cell tumours. This knowledge can be utilised to develop targeted antitumor therapies aimed at selectively inhibiting aberrant NF-κB activity.
The non-canonical NF-κB signalling pathway has been implicated in the expansion and lymphomagenesis of CD40-activated B cells. In a mouse model, constitutive CD40 activation led to the selective activation of the non-canonical NF-κB pathway, suggesting a potential role for RelB-regulated genes in lymphoma development. However, the specific contribution of these genes to lymphoma development remains to be fully elucidated.
Planning a Visit to the National Constitution Center?
You may want to see also

Genetic mutations and the constitutive activation of NF-κB
The NF-κB transcription factor family is crucial for lymphocyte proliferation and survival. Genetic mutations or aberrant expression of proteins that control NF-κB signalling can lead to constitutive activation of NF-κB, promoting tumour growth and survival. This is evident in various lymphoid malignancies, including diffuse large B-cell lymphoma, Hodgkin lymphoma, and adult T-cell leukemia.
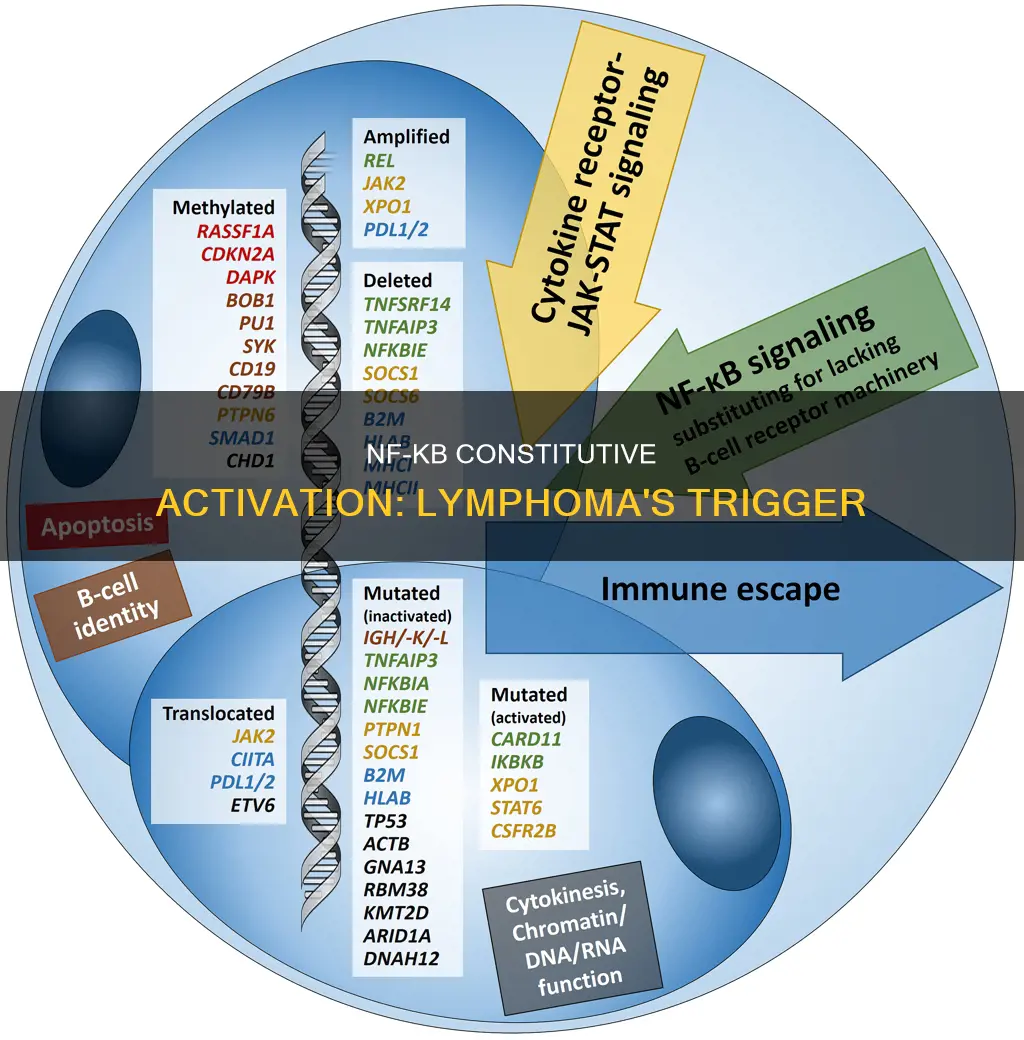
Genetic mutations in NF-κB pathway components can result in the constitutive activation of the canonical or alternative pathway. For instance, mutations in the IKBA coding region or the IkappaBalpha gene can contribute to the constitutive activation of NF-κB in activated B cell-like diffuse large B-cell lymphoma. Additionally, the genetic inactivation of A20, a negative regulator of IKK activation, has been observed in several lymphoid malignancies, including Hodgkin lymphoma and MALT lymphoma. The loss of A20 function alone may not be sufficient for lymphomagenesis, but it is often associated with additional genetic aberrations, such as MYD88 or CD79A/B mutations, that drive constitutive NF-κB activation.
The API2-MALT1 fusion oncoprotein, formed by the recurrent t(11;18)(q21;q21) translocation in mucosa-associated lymphoid tissue (MALT) lymphoma, induces proteolytic cleavage of NF-κB-inducing kinase (NIK). This results in deregulated NIK activity, leading to constitutive non-canonical NF-κB signalling, enhanced B cell adhesion, and apoptosis resistance.
Furthermore, studies have shown that RelB-regulated genes contribute to B cell lymphomagenesis. In a mouse model with a constitutively active CD40 receptor in B cells, the chronic activation of CD40 signalling led to the selective activation of the non-canonical NF-kB pathway, promoting the expansion and lymphomagenesis of CD40-activated B cells.
Understanding the specific genetic mutations and their impact on NF-κB activation is essential for developing targeted antitumor therapies that selectively inhibit aberrant NF-κB activity and improve lymphoma treatment.
Celebrating Constitution Day: Mexican Style!
You may want to see also
Explore related products
$24.99 $26.99

NF-κB and the survival of activated B cell-like diffuse large B-cell lymphoma cells
NF-κB is a regulator of genes that control cell proliferation and cell survival. In cancer, proteins that control NF-κB signalling are mutated or aberrantly expressed, leading to a loss of coordination between the malignant cell and the rest of the organism. This is evident in metastasis and in the immune system's inability to eradicate the tumour.
The NF-κB transcription factor family plays a crucial role in lymphocyte proliferation and survival. Aberrant NF-κB activation has been observed in several lymphoid malignancies, including diffuse large B-cell lymphoma, Hodgkin lymphoma, and adult T-cell leukemia. Persistent infections, a pro-inflammatory microenvironment, self-reactive immune receptors, and genetic lesions that alter the function of key signalling effectors are all factors that contribute to constitutive NF-κB activity in these malignancies.
In B-cell malignancies, the NF-κB pathway is frequently constitutively activated due to multiple mutations in pathway components. This activation is essential for the survival of activated B cell-like diffuse large B-cell lymphoma cells.
The non-canonical NF-κB pathway has been shown to contribute to the expansion and lymphomagenesis of CD40-activated B cells. In a mouse model with a constitutively active CD40 receptor in B cells, the selective activation of the non-canonical NF-κB pathway was observed. However, the role of RelB-regulated genes in lymphoma development is still unclear. While RelB is important for the viability of a Hodgkin Lymphoma cell line, its inactivation in mature B cells had only minor effects on B cell maintenance and activation.
The API2-MALT1 fusion oncoprotein, which is commonly found in mucosa-associated lymphoid tissue (MALT) lymphoma, induces proteolytic cleavage of NF-κB-inducing kinase (NIK), resulting in constitutive non-canonical NF-κB signalling, enhanced B cell adhesion, and apoptosis resistance.
Bid Acceptance: When Does It Become a Binding Contract?
You may want to see also

NF-κB activation in lymphoid malignancies
NF-κB activation is a crucial process in the proliferation and survival of lymphocytes. Its aberrant activation has been observed in several lymphoid malignancies, including diffuse large B-cell lymphoma, Hodgkin lymphoma, and adult T-cell leukemia. This activation is influenced by various factors, such as persistent infections, a pro-inflammatory cancer microenvironment, self-reactive immune receptors, and genetic lesions affecting key signalling effectors.
The NF-κB signalling pathway is complex, involving both canonical and non-canonical pathways. The canonical pathway is primarily activated through the B-cell receptor (BCR) and CD40, leading to the translocation of NF-κB subunits into the nucleus. In the non-canonical pathway, activation of RelB contributes to the survival and activation of B cells, with its role in lymphoma development being a subject of ongoing research.
Genetic mutations in NF-κB pathway components can lead to constitutive activation of either the canonical or non-canonical pathway. For instance, mutations in the TNFAIP3 gene, encoding A20, can result in partial or complete loss of its negative regulatory function in lymphoid malignancies. Additionally, the API2-MALT1 fusion oncoprotein, associated with mucosa-associated lymphoid tissue (MALT) lymphoma, induces proteolytic cleavage of NF-κB-inducing kinase (NIK), resulting in constitutive non-canonical NF-κB signalling.
Understanding the specific genetic mutations and oncogenic mechanisms driving NF-κB activation in lymphoid malignancies is essential for developing targeted therapies. By analysing the relevant oncogenic lesions and deregulated signalling pathways, more effective lymphoma treatments can be designed, such as simultaneously targeting complementary signalling pathways.
In summary, constitutive activation of NF-κB contributes to lymphomagenesis by promoting the growth and survival of malignant cells. The complex interplay between genetic mutations, signalling pathways, and cellular functions makes it crucial to comprehend the distinct roles of NF-κB transcription factors to develop targeted therapeutic interventions.
Iroquois Constitution's Influence on the Declaration of Independence
You may want to see also
Frequently asked questions
NF-κB is a regulator of genes that control cell proliferation and survival in lymphocytes.
Constitutive activation of NF-κB leads to the survival and proliferation of malignant cells, resulting in lymphomagenesis.
Factors such as persistent infections, the pro-inflammatory microenvironment of the cancer, self-reactive immune receptors, and genetic lesions contribute to constitutive activation.
Aberrant activation of NF-κB promotes tumorigenesis by allowing the malignant cell to bypass affinity selection and recirculate between DZ and LZ in an uncontrolled manner.
RelB-regulated genes contribute to the survival and activation of B cells, which are chronically activated by CD40 signaling, leading to lymphomagenesis.










































