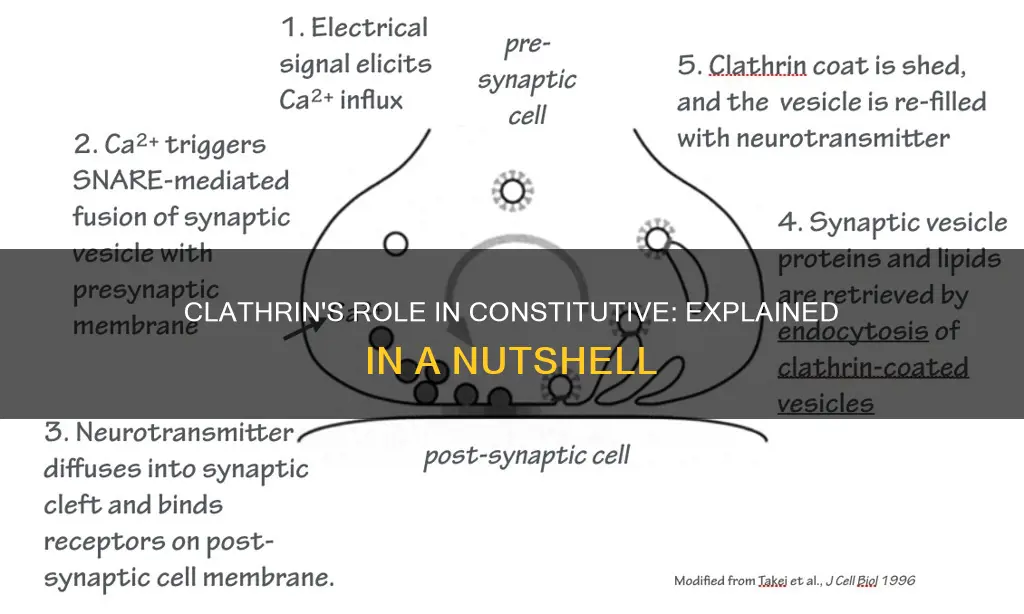
Clathrin is a protein that plays a crucial role in the internalization of various molecules and cargo packaging into vesicles. This process, known as clathrin-mediated endocytosis, is essential for several physiological functions in higher eukaryotes, including signal termination, exocytosis, and neurotransmission. Clathrin forms a triskelion structure composed of three heavy chains and three light chains, which facilitate the formation of clathrin-coated vesicles. While clathrin-dependent endocytosis is prominent in yeast and animals, its role in plants has been less defined. In this regard, studies have demonstrated the involvement of clathrin in the constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. However, certain processes, such as the recycling of uPAR, have been found to occur through a pathway independent of clathrin.
| Characteristics | Values |
|---|---|
| Clathrin-mediated endocytosis | Essential for many physiological processes in higher eukaryotes, including signal termination and exocytosis |
| Clathrin-coated pits | Found at the location of phagosomes and surface adhesion sites of macrophages |
| Clathrin-coated vesicles | Clathrin forms the coat of purified coated vesicles |
| Clathrin-dependent endocytosis | Involves core and accessory adaptor proteins that package cargoes into vesicles |
| Clathrin light chains | Regulate the formation and disassembly of a clathrin lattice |
| Clathrin heavy chains | Provide the structural backbone of the clathrin lattice |
| Clathrin triskelion | Composed of three clathrin heavy chains and three light chains that form a subunit that polymerizes to form a clathrin-coated vesicle |
| Clathrin function | Involved in the intracellular trafficking of a wide range of cargo |
Explore related products
$91.96 $109.99
What You'll Learn

Clathrin-mediated endocytosis in insects
Clathrin-mediated endocytosis (CME) is a key process in vesicular trafficking that transports a wide range of cargo molecules from the cell surface to the interior. It is the main mechanism for internalization of cell-surface molecules and surface-bound cargoes. Clathrin-mediated endocytosis was first described over 50 years ago, and since then, over 50 proteins have been shown to be part of the molecular machinery that generates the
In insects, clathrin-mediated endocytosis is particularly important for the entry of viruses into the cells of their arthropod vectors. It is also likely that it plays a similar role in receptor internalization as in vertebrates. During clathrin-mediated endocytosis in insects, a coated pit is formed at the inner surface of the plasma membrane, which buds into the cell to form clathrin-coated vesicles that can fuse with endosomes. The actual budding-in process, whereby a clathrin-coated pit is converted into a vesicle, is regulated by clathrin, assisted by cytoplasmic proteins, including the adaptor protein AP2 and dynamin.
Clathrin triskelion in the cytoplasm binds to an adaptor protein that has bound to the membrane, linking one of its three feet to the membrane at a time. Clathrin cannot bind to the membrane or cargo directly and instead uses adaptor proteins to do this. This triskelion will bind to other membrane-attached triskelia to form a rounded lattice of hexagons and pentagons, reminiscent of the panels on a soccer ball, that pulls the membrane into a bud. By constructing different combinations of 5- and 6-sided rings, vesicles of different sizes may assemble.
In pest insects, dsRNAs are being developed as novel biocidal molecules that target essential genes. In most insect control applications, the dsRNAs are intended to be incorporated into or sprayed on their food source, and hence, uptake of the dsRNA will occur through the intestinal cells. In some species of beetles, SID1-like (SIL) proteins have been identified, but their role in dsRNA uptake is unclear, as knockouts of these genes in some species had no impact on RNAi efficacy.
Understanding the Framework: Constitution's Key Components
You may want to see also

Clathrin-coated vesicles
Clathrin is a cytosolic protein that plays a role in the intracellular trafficking of a wide range of cargo. It is composed of three heavy chains and three light chains that form a clathrin triskelion, which is the subunit that polymerizes to form a clathrin-coated vesicle. Clathrin-coated vesicles are involved in various cellular and biological processes, including membrane trafficking, chromosomal segregation during mitosis, and organelle biogenesis.
The clathrin triskelion has three heavy chains that provide the structural backbone of the clathrin lattice, and three light chains that regulate the formation and disassembly of the lattice. The heavy chains are located on chromosomes 17 and 22 in humans, with the former being found in all cells and the latter expressed in muscle. The light chains bind primarily to the proximal leg portion of the heavy chain, with some interaction near the trimerization domain.
Understanding the Constitution: Amendments Explained
You may want to see also

Clathrin light chains
Clathrin is a major coat protein that plays a role in sorting and retaining proteins at the late Golgi and in endocytosis from the cell surface. The clathrin triskelion contains three heavy chains and three light chains. The heavy chains form the structural backbone of the clathrin lattice, while the light chains are thought to regulate the formation and disassembly of the clathrin lattice.
The clathrin light chain (CLC) subunits participate in several membrane traffic pathways involving both clathrin and actin, through binding the actin-organizing huntingtin-interacting proteins (Hip). However, CLCs are not always necessary for clathrin-mediated endocytosis of many cargoes. CLC depletion affects cell migration through Hip binding and reduces the surface expression of β1-integrin by interfering with recycling following normal endocytosis of inactive β1-integrin.
The light chains bind primarily to the proximal leg portion of the heavy chain, with some interaction near the trimerization domain. The β-propeller at the 'foot' of clathrin contains multiple binding sites for interaction with other proteins. The light chain can function independently of the clathrin heavy chain, as seen in yeast.
Checks and Balances: Constitutional Cornerstone?
You may want to see also

Clathrin and LRP-1-independent constitutive endocytosis
Clathrin-mediated endocytosis is a process where clathrin forms a coat around purified coated vesicles. It is essential for many physiological processes in higher eukaryotes, including signal termination and exocytosis. Clathrin-mediated endocytosis is the endocytic portal into cells through which cargo is packaged into vesicles with the aid of a clathrin coat.
The study found that in addition to the uPA:PAI-1- and LRP-1-mediated route, there exists an alternative, independent pathway for uPAR internalization. This pathway is uPA:PAI-1-independent, lipid rafts-independent, and amiloride-sensitive. It is a macropinocytic-like process associated with the rapid recycling of uPAR to the cell surface.
The study utilized two cell lines: HEK293-uPAR, which does not produce uPA or PAI-1 and therefore cannot undergo clathrin-dependent endocytosis, and HT1080, a human fibrosarcoma line that expresses high levels of uPAR, uPA, and PAI-1, and thus undergoes clathrin-dependent endocytosis. Despite both lines expressing LRP-1, the spatial segregation of endocytic markers and the absence of co-localization suggest a clathrin- and LRP-1-independent mechanism for constitutive uPAR endocytosis.
In summary, while clathrin-mediated endocytosis is a well-studied process, certain scenarios, such as the endocytosis of uPAR, exhibit a clathrin- and LRP-1-independent mechanism, highlighting the complexity and diversity of endocytic pathways.
Federalist Papers: Constitution's Companion or Something More?
You may want to see also

Clathrin-dependent endocytosis
Clathrin is a cytosolic protein composed of three heavy chains and three light chains that form a triskelion, which is the subunit that polymerises to form a clathrin-coated vesicle. The three heavy chains provide the structural backbone of the clathrin lattice, while the three light chains regulate the formation and disassembly of the lattice. The light chains bind primarily to the proximal leg portion of the heavy chain, with some interaction near the trimerization domain. The β-propeller at the 'foot' of clathrin contains multiple binding sites for interaction with other proteins.
Clathrin-coated vesicles are formed when the clathrin triskelion binds to an adaptor protein that has bound to the membrane, linking one of its three feet to the membrane at a time. The triskelion then binds to other membrane-attached triskelia to form a rounded lattice of hexagons and pentagons, pulling the membrane into a bud. Different combinations of 5-sided and 6-sided rings allow for vesicles of different sizes to assemble. The smallest clathrin cage commonly imaged, called a mini-coat, has 12 pentagons and only two hexagons.
Clathrin-mediated endocytosis is a modular process that involves core and accessory adaptor proteins that package cargoes into vesicles, ultimately leading to their uptake. It is essential for many physiological processes in higher eukaryotes, including signal termination and exocytosis, and is fundamental to neurotransmission, signal transduction, and the regulation of plasma membrane activities.
Understanding the Executive Branch: What's Excluded?
You may want to see also
Frequently asked questions
Clathrin is a cytosolic protein that is involved in the intracellular trafficking of a wide range of cargo. It is made up of three heavy chains and three light chains that form a triskelion, which is the subunit that polymerizes to form a clathrin-coated vesicle.
Clathrin-mediated endocytosis is a process where clathrin forms a coat around a vesicle, aiding in the packaging and delivery of cargo. This process is essential for many physiological processes in higher eukaryotes, including signal termination and exocytosis.
Clathrin has been found to play a role in constitutive endocytosis in some cases, such as in plants and with specific proteins like PIN auxin efflux carriers. However, there are also instances where clathrin-independent constitutive endocytosis has been observed, such as with uPAR.