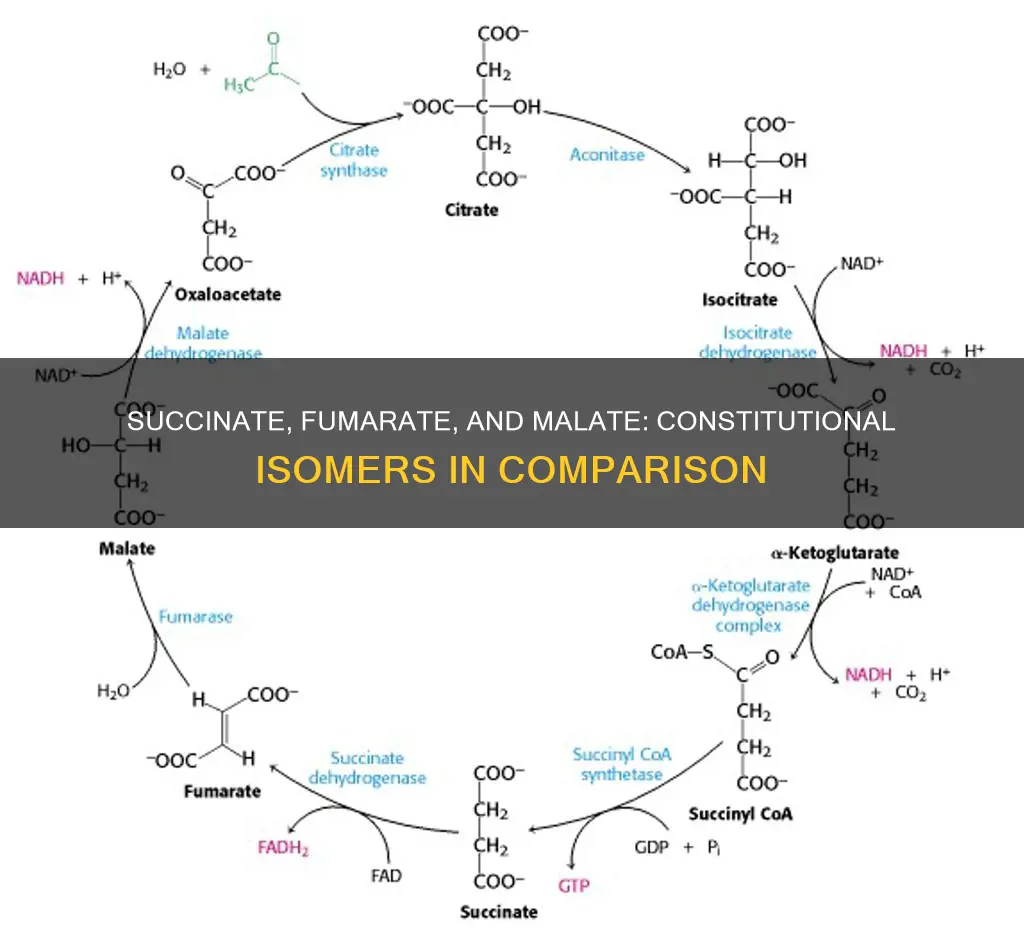
In organic chemistry, isomers are two or more molecules that share the same molecular formula but differ in the way their constituent atoms are connected. There are several types of isomers, including constitutional isomers, stereoisomers, enantiomers, and diastereomers. This paragraph aims to explore the relationship between succinate, fumarate, and malate to determine if they are constitutional isomers of each other.
| Characteristics | Values |
|---|---|
| Definition of constitutional isomers | Compounds that differ in connectivity, i.e., in the way in which the constituent atoms are connected to one another |
| Definition of succinate | A compound formed during the metabolic process of mixed acid fermentation |
| Definition of fumarate | A compound formed during the metabolic process of mixed acid fermentation |
| Definition of malate | A compound formed during the metabolic process of mixed acid fermentation |
| Relationship between succinate and fumarate | Succinate is converted to fumarate by succinate dehydrogenase |
| Relationship between fumarate and malate | Fumarate is converted to malate by the enzyme fumarase |
| Relationship between succinate and malate | Succinate and malate are both substrates of the SDH enzyme |
Explore related products
$17.99 $19.99
What You'll Learn

Fumarate and fumarate reduction
Fumarate reductase is a membrane-bound enzyme that catalyses the reduction of fumarate to succinate. It is a multimeric protein that can also catalyse the reverse reaction, succinate oxidation, which is usually catalysed by succinate dehydrogenase (SDH). Both enzymes share several similarities, including their activity, cofactor, and subunits' composition. Fumarate reductase is closely related to succinate dehydrogenases, which are central to bacterial and eukaryotic respiratory chains, as well as the tricarboxylic acid (TCA) cycle.
The fumarate hydratase gene, FH, encodes two fumarate isoenzymes: cytosolic and mitochondrial. FH catalyses the conversion of fumarate to malate in the TCA cycle. Mutations in the FH gene can lead to the accumulation of fumarate and other TCA intermediates, which has been associated with various cancers and diseases.
Fumarate reductases enable bacteria to respire anaerobically using fumarate as a terminal electron acceptor. They are important in microbial metabolism and are differentially regulated by ArcBA and FNR in bacterial cells. Fumarate reductase couples the reduction of fumarate to succinate with the oxidation of quinol to quinone, a reaction opposite to that catalysed by succinate dehydrogenase. This process is essential for colonization in the mouse intestine under anoxic conditions.
Northwest Ordinance: Forging the US Constitution's Federal Framework
You may want to see also

Fumarate hydratase gene
Succinate, fumarate, and malate are constitutional isomers of each other. Constitutional isomers are compounds that differ in the way in which the constituent atoms are connected to one another.
Now, onto the fumarate hydratase gene. The fumarate hydratase gene, also known as FH, spans 22kb and contains ten exons. This gene encodes two fumarate isoenzymes: cytosolic and mitochondrial. FH is a crucial enzyme that facilitates the conversion of fumarate to malate in the tricarboxylic acid (TCA) cycle, also known as the Krebs cycle.
The active form of FH is a homotetramer, where three out of the four chains unite to form the enzymatic active site. When FH is lost, there is an accumulation of fumarate and other TCA intermediates. Mutations in the FH gene have been linked to various health conditions, including hereditary leiomyomatosis and renal cell cancer (HLRCC), an autosomal dominant disorder characterised by smooth muscle tumours of the skin, uterus, and/or renal cancer. These mutations can lead to the absence or truncation of proteins or substitutions and deletions of highly conserved amino acids.
Fumarate hydratase deficiency (FHD) is another condition associated with mutations in the FH gene. FHD is an autosomal recessive disorder characterised by neurological impairment and encephalopathy. The impact of FHD is particularly notable in the brain, where only the mitochondrial isoenzyme has been identified, offering a potential explanation for the predominant effect on brain function.
In summary, the fumarate hydratase gene plays a vital role in the TCA cycle by encoding for fumarate isoenzymes. Mutations in this gene can have significant health consequences, including the development of tumours and neurological impairments.
Exploring the USS Constitution: A Visitor's Guide
You may want to see also

Fumarase and its role in the primary metabolism
Fumarase, also known as fumarate hydratase (FH), is a metabolic enzyme that plays a critical role in the tricarboxylic acid (TCA) cycle, or Krebs cycle. The TCA cycle is a series of chemical reactions that occur in living cells to generate energy and facilitate various metabolic processes. Fumarase, specifically, catalyses the conversion of fumarate to malate in the TCA cycle.
Fumarase exists in two forms: the mitochondrial form and the cytosolic form. The mitochondrial form of fumarase is involved in energy production through its participation in the TCA cycle. This form of fumarase is particularly important in plant systems, where it plays a role in carbon storage and utilization. For example, in Arabidopsis, fumarate serves as an alternative carbon sink for photosynthate, similar to starch, indicating its significance in primary metabolism. Additionally, fumarate has been identified in soybean and alfalfa roots, although in very low concentrations.
On the other hand, the cytosolic form of fumarase is recruited from the cytosol to the nucleus upon DNA damage induction. This form plays a crucial role in protecting cells from DNA damage, especially DNA double-strand breaks. When DNA damage occurs, the absence of fumarase in cells can be compensated for by high concentrations of fumaric acid, one of the products of the fumarase enzyme. This discovery highlights the connection between primary metabolism and the cell's response to DNA damage, providing insights into the control of tumor propagation.
Furthermore, fumarase is also implicated in anaerobic mitochondrial respiration. In normal cells, such as those found in freshly isolated kidney proximal tubules, fumarate, in combination with α-keto glutarate and malate, can promote anaerobic respiration, with succinate as the end product. This process is particularly relevant under anoxic conditions, as it provides an alternative pathway for energy production when oxygen is limited.
In summary, fumarase, through its mitochondrial and cytosolic forms, contributes to primary metabolism by facilitating energy production, responding to DNA damage, and providing an alternative respiratory pathway under anaerobic conditions. These functions highlight the importance of fumarase in maintaining cellular homeostasis and protecting against diseases such as cancer.
The US Constitution: Its Core Principles and Values
You may want to see also
Explore related products

Succinate and its formation
Succinate, or succinic acid, is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. It is a key intermediate in the tricarboxylic acid (TCA) cycle, a primary metabolic pathway used to produce chemical energy in the presence of oxygen. Succinate is generated from succinyl-CoA by the enzyme succinyl-CoA synthetase in a GTP/ATP-producing step. It is then oxidised by the enzyme succinate dehydrogenase (SDH) to form fumarate, which is involved in making ATP. This process is important in cellular metabolism, particularly ATP formation, and the regulation of cellular function.
The formation of succinate through the TCA cycle is just one method of its production. It can also be formed through the GABA (gamma-aminobutyric acid) shunt, which acts as an alternative route to convert alpha-ketoglutarate into succinate, bypassing the TCA cycle intermediate succinyl-CoA. Transamination and subsequent decarboxylation of alpha-ketoglutarate lead to the formation of GABA, which is then metabolised by GABA transaminase to succinic semialdehyde. This is then oxidised by succinic semialdehyde dehydrogenase (SSADH) to form succinate, which re-enters the TCA cycle.
Succinate is also produced through mixed acid fermentation, a metabolic process by which a six-carbon sugar (e.g. glucose) is converted into a complex and variable mixture of acids. This process is anaerobic and common in bacteria, particularly E. coli. The end products of mixed acid fermentation include lactate, acetate, succinate, formate, ethanol, and carbon dioxide.
Furthermore, succinate can be formed through the reduction of fumarate by the enzyme fumarate reductase. This reaction is important in the metabolism of facultatively anaerobic Enterobacteriaceae and is essential for the colonisation of the mouse intestine.
Finally, succinate can be produced synthetically and is generally recognised as safe by the U.S. Food and Drug Administration. It is used as an acidity regulator and flavouring agent in the food and beverage industry, as well as an excipient in pharmaceutical products.
Who is a Citizen? Black People and the US Constitution
You may want to see also

Malate and its role in the TCA cycle
Malate, or (S)-malate, is a key component of the tricarboxylic acid (TCA) cycle, also known as the citric acid cycle. The TCA cycle is a series of biochemical reactions that occur in living cells to release stored energy in the form of adenosine triphosphate (ATP). Malate is an important intermediate in this cycle, playing a role in several metabolic processes and influencing various cellular functions.
The TCA cycle involves the oxidation of acetyl-CoA, which produces carbon dioxide and water, with the released energy captured as ATP. During the cycle, citrate is converted into isocitrate, which is then converted into α-ketoglutarate and subsequently into succinyl-CoA. This succinyl-CoA is then converted into succinate, which is oxidized to generate fumarate. At this stage, fumarate is converted into malate, which is then further converted into oxaloacetate (OAA). This OAA combines with another acetyl-CoA molecule to continue the TCA cycle.
Malate, as an intermediate in the TCA cycle, is involved in several metabolic processes. For instance, during gluconeogenesis, mitochondrial oxaloacetate is reduced to malate, which is then transported out of the mitochondrion and oxidized back to oxaloacetate in the cytosol. This oxaloacetate is crucial for the synthesis of glucose in the liver and kidney. Additionally, malate is involved in the conversion of pyruvate to oxaloacetate, which increases the cycle's capacity to metabolize acetyl-CoA when energy demands are high, such as during muscle activity.
The TCA cycle and its intermediates, including malate, have been found to influence multiple cellular functions beyond biosynthesis. Recent evidence suggests that TCA cycle intermediates act as signaling molecules, controlling processes such as chromatin modifications, DNA methylation, the hypoxic response, and immunity. Furthermore, the TCA cycle has been associated with certain diseases. For example, mutations in the fumarate hydratase (FH) gene, which encodes an enzyme that catalyzes the conversion of fumarate to malate, have been linked to various cancers and diseases.
In summary, malate is a crucial intermediate in the TCA cycle, playing a role in multiple metabolic processes and influencing various cellular functions. The TCA cycle itself is a complex and dynamic system that is involved in energy production, biosynthesis, and the control of cellular processes, with malate serving as one of its key constituents.
Patrick Henry's Constitution: His Vision and Legacy
You may want to see also
Frequently asked questions
Constitutional isomers are compounds that differ in the way in which their constituent atoms are connected to one another.
No, succinate, fumarate, and malate are not constitutional isomers of each other. However, they are related. Fumarase is the enzyme that catalyzes the addition of water to fumaric acid to form S-malate. Fumarate can also be converted to succinate by succinate dehydrogenase.
They are stereoisomers, which have the same connectivity but differ in the way in which the constituent atoms are oriented in space.
Examples of constitutional isomers include cyclohexane and 1-hexene, which both have the molecular formula C6H12 but differ in connectivity.























![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 100 Milligrams](https://m.media-amazon.com/images/I/71-dKlpNLrS._AC_UL320_.jpg)
![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 50 Milligrams](https://m.media-amazon.com/images/I/81HAPnASH5L._AC_UL320_.jpg)
![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 250 Milligrams](https://m.media-amazon.com/images/I/71EL45ccGFS._AC_UL320_.jpg)

















