In chemistry, isomers are compounds with the same number and type of atoms but differ in the way the atoms are connected. They can be further classified into two broad classes: constitutional isomers and stereoisomers. Constitutional isomers, also known as structural isomers, differ in their connectivity or the arrangement of bonds between atoms. Stereoisomers, on the other hand, have the same connectivity but differ in the arrangement of atoms in space. Now, when it comes to the terms ortho, meta, and para, they are used to describe the specific relative locations of substituents on a disubstituted aromatic ring. The prefixes ortho, meta, and para are derived from Greek, meaning correct, following, and beside, respectively. With this background information, we can now explore and answer the question: Are ortho, meta, and para positions examples of constitutional isomerism?
| Characteristics | Values |
|---|---|
| Definition | Ortho, meta, and para are all derived from Greek, meaning correct, following, and beside, respectively. |
| Ortho | Refers to the 1,2 position |
| Meta | Refers to the 1,3 position |
| Para | Refers to the 1,4 position |
| Examples | Ortho isomer catechol, meta isomer resorcinol, and para isomer hydroquinone are the three arene substitution isomers of dihydroxybenzene (C6H4(OH)2). |
| Ortho isomer phthalic acid, meta isomer isophthalic acid, and para isomer terephthalic acid are the three arene substitution isomers of benzenedicarboxylic acid (C6H4(COOH)2). | |
| Ortho-, meta-, and para-directors are terms used in Electrophilic Aromatic Substitution (EAS) to describe the positioning of substituents on a benzene ring. | |
| Electron-donating groups, such as amino, hydroxyl, alkyl, and phenyl groups, are typically ortho/para-directors, while electron-withdrawing groups, such as nitro, nitrile, and ketone groups, are usually meta-directors. | |
| Separation | Column chromatography and fractional crystallisation are methods used to separate ortho, meta, and para isomers. |
Explore related products
What You'll Learn
- Ortho, meta, and para are types of constitutional isomers
- Constitutional isomers are compounds that differ in connectivity
- Ortho, meta, and para positions are determined by how well a substituent stabilises an adjacent carbocation
- Electron donating groups are ortho/para-directors, electron withdrawing groups are meta-directors
- Ortho, meta, and para positions are relative locations of substituents on a disubstituted aromatic ring

Ortho, meta, and para are types of constitutional isomers
Ortho, meta, and para are, indeed, types of constitutional isomers. Constitutional isomers are compounds that differ in connectivity, meaning that the way in which the constituent atoms are connected to one another varies. In other words, they are structural isomers, which have the same number of atoms of each element but differ in the bonds between them.
The prefixes ortho, meta, and para are derived from Greek, meaning "correct", "following", and "beside", respectively. These terms are used to distinguish isomers of disubstituted aromatic rings, with ortho relating to the 1,2 position, meta to the 1,3 position, and para to the 1,4 position. For example, there are three arene substitution isomers of dihydroxybenzene (C6H4(OH)2): the ortho isomer catechol, the meta isomer resorcinol, and the para isomer hydroquinone.
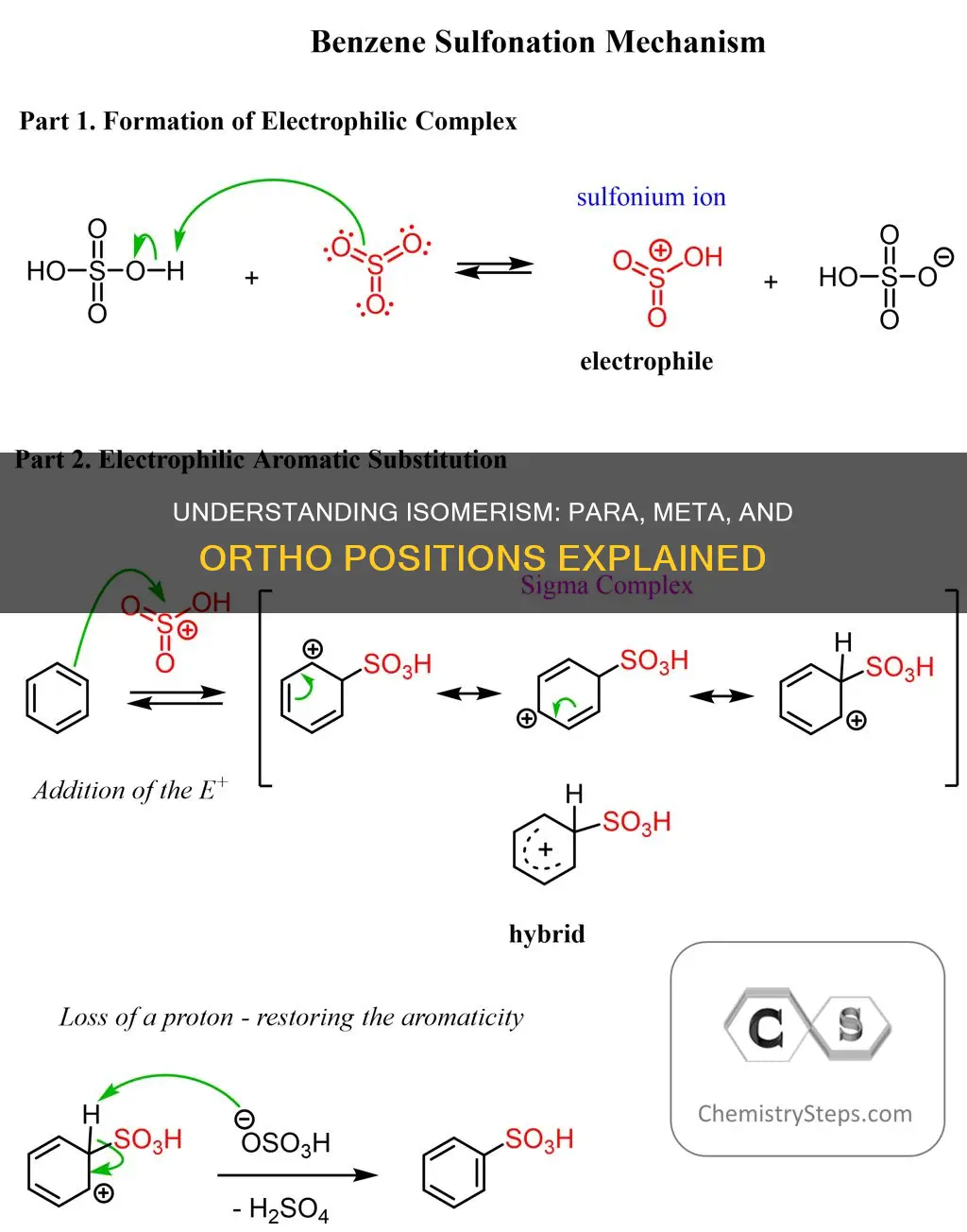
In Electrophilic Aromatic Substitution (EAS), some substituents on benzene will direct the electrophile to the ortho and para positions. These are called "ortho, para-directors". Another class of substituents avoids directing the electrophile to those positions, resulting in the formation of the meta-product. Electron-donating groups, such as amino, hydroxyl, alkyl, and phenyl groups, tend to be ortho/para-directors, while electron-withdrawing groups like nitro, nitrile, and ketone groups tend to be meta-directors.
The separation of ortho and para isomers can be challenging in synthetic chemistry due to their similar boiling points. However, the para isomer typically exhibits the highest melting point and lowest solubility in a given solvent among the three isomers. Techniques like column chromatography and fractional crystallization can be employed to separate these isomers effectively.
Cabinet-Level Positions: Understanding the Key Duo
You may want to see also

Constitutional isomers are compounds that differ in connectivity
In chemistry, constitutional isomers, also known as structural isomers, are compounds that differ in their connectivity or the way in which their constituent atoms are connected to one another. They have the same molecular formula, but their internal structure, or the bonds between their atoms, differs. An example of this is the three arene substitution isomers of dihydroxybenzene (C6H4(OH)2): the ortho isomer catechol, the meta isomer resorcinol, and the para isomer hydroquinone.
The prefixes ortho, meta, and para, derived from the Greek words for "correct", "following", and "beside" respectively, are used to distinguish isomers of disubstituted aromatic rings. In the context of Electrophilic Aromatic Substitution (EAS), ortho and para directors are substituents on benzene that direct the electrophile E to the ortho (1,2) and para (1,4) positions, respectively. On the other hand, meta-directors are substituents that avoid directing the electrophile E to the ortho or para positions, resulting in the formation of the meta-product (1,3).
The specific behaviour of these directors depends on the compound in question. However, electron-donating groups such as amino, hydroxyl, alkyl, and phenyl groups tend to be ortho/para-directors, while electron-withdrawing groups such as nitro, nitrile, and ketone groups tend to be meta-directors. For instance, in the electrophilic aromatic substitution of methoxybenzene, the para-product dominates (60-70%), with the ortho-product close behind (30-40%), and only a trace of the meta-product.
The concept of constitutional isomerism is distinct from stereoisomerism, where the atoms and bonding scheme remain the same, but the relative spatial arrangement of the atoms differs.
C-Way Quota Utilization: Actionable Steps for Effective Implementation
You may want to see also

Ortho, meta, and para positions are determined by how well a substituent stabilises an adjacent carbocation
Ortho, meta, and para positions are determined by how effectively a substituent stabilises an adjacent carbocation. This is known as electrophilic aromatic substitution (EAS). In EAS, some substituents on benzene direct the electrophile to the ortho (1,2) and para (1,4) positions. These are called "ortho, para directors".
Substituents that have a lone pair on the atom adjacent to the aromatic ring will be ortho, para directors since they can form a new pi-bond with an adjacent carbocation. Groups such as hydroxyl, alkyl, and phenyl are examples of electron-donating groups that act as ortho, para directors.
Another class of substituents avoids directing the electrophile to the ortho or para positions, resulting in the meta (1,3) product being major. These are called "meta directors". Substituents that pull electron density away from adjacent carbocations, such as CF3 and NO2, will direct the electrophile to the meta position instead.
The ratio of ortho to para substitution can be difficult to predict. The para position is favoured due to the steric hindrance of the ortho position. However, there are two ortho positions and only one para, which increases the yield of the ortho substitution. The larger the group on the ring, the more favoured the para substitution will be.
The terms ortho, meta, and para are derived from Greek, meaning "correct", "following", and "beside", respectively. These terms were first used to distinguish isomers of disubstituted aromatic rings by Wilhelm Körner in 1867, although he applied the prefixes differently than they are used today.
The Path to US Citizenship: 9-Year Journey
You may want to see also
Explore related products

Electron donating groups are ortho/para-directors, electron withdrawing groups are meta-directors
The terms "ortho", "meta", and "para" refer to the relative locations of substituents on a disubstituted aromatic ring. These terms were first used by German chemist Karl Gräbe in 1869. The ortho description was historically used to designate the original compound, the meta description for isomers, and the para description for closely related compounds.
In Electrophilic Aromatic Substitution (EAS), some substituents on benzene direct the electrophile E to the ortho (1,2) and para (1,4) positions. These are called "ortho, para-directors" or "ortho/para directors". Ortho/para directors are typically electron-donating groups, such as amino, hydroxyl, alkyl, and phenyl groups. Examples of ortho-, para- directors are hydroxyl groups, ethers, amines, alkyl groups, thiols, and halogens.
Another class of substituents avoids directing the electrophile E to the ortho or para positions, resulting in the formation of the meta- product (1,3) as the major product. These are called "meta-directors". Meta-directors are typically electron-withdrawing groups, such as nitro, nitrile, and ketone groups. Examples of meta-directors include nitriles, carbonyl compounds (such as aldehydes, ketones, and esters), sulfones, electron-deficient alkyl groups, nitro groups, and alkylammoniums.
The ultimate factor that determines whether a substituent is ortho-, para-, or meta-directing is how well it stabilizes an adjacent carbocation. Substituents with a lone pair on the atom adjacent to the aromatic ring will be ortho-, para- directors since they can form a new pi-bond with an adjacent carbocation. Substituents that pull electron density away from adjacent carbocations will avoid directing E to the ortho or para position, resulting in the formation of the meta- product.
The specific substitution pattern of ortho, meta, and para isomers is an example of positional isomerism, a type of constitutional isomerism. Constitutional isomers are compounds that differ in connectivity, i.e., the way in which the constituent atoms are connected to one another. Positional isomers have the same functional groups but differ in their location within the molecule.
George Mason's Constitutional Vision: Freedom and Rights
You may want to see also

Ortho, meta, and para positions are relative locations of substituents on a disubstituted aromatic ring
The terms ortho, meta, and para refer to the relative locations of substituents on a disubstituted aromatic ring. These terms were first used by German chemist Karl Gräbe in 1869 to denote specific relative locations of substituents on a disubstituted aromatic ring, specifically naphthalene. The ortho description was historically used to designate the original compound, with meta referring to the isomer, and para reserved for closely related compounds.
In the context of aromatic substitution, these terms describe the positions of substituents on an aromatic ring. The ortho isomer refers to a substituent located at the 1,2 position, the meta isomer to the 1,3 position, and the para isomer to the 1,4 position. These positions are determined by the number of carbon atoms separating the substituents. For example, in the case of dichlorobenzene, the second substitution of hydrogen by chlorine can yield three positional isomers: 1,2- or ortho-, 1,3- or meta-, and 1,4- or para-dichlorobenzene.
The prefixes ortho, meta, and para are derived from Greek, meaning "correct," "following," and "beside," respectively. In Electrophilic Aromatic Substitution (EAS), some substituents on benzene will direct the electrophile to the ortho and para positions, while another class of substituents avoids directing the electrophile to those positions, resulting in the formation of the meta product. Electron-donating groups, such as amino, hydroxyl, alkyl, and phenyl groups, tend to be ortho/para-directors, while electron-withdrawing groups, such as nitro, nitrile, and ketone groups, tend to be meta-directors.
The specific substitution pattern can have a significant impact on the properties of the resulting isomers. For example, in simple disubstituted arenes, the three isomers tend to have similar boiling points, but the para isomer usually has the highest melting point and the lowest solubility in a given solvent. The relative positions of substituents on an aromatic ring can also influence the reactivity and stability of the molecule, as well as its physical properties such as boiling point, melting point, and solubility.
The Constitution's Ratification: A Historical Turning Point
You may want to see also
Frequently asked questions
Constitutional isomers are compounds that differ in connectivity, i.e., the way in which the constituent atoms are connected to one another.
Yes, ortho, meta, and para are examples of positional isomers, a type of constitutional isomer where the functional groups are the same but differ in their location within the molecule.
Some examples of ortho, meta, and para isomers include the three arene substitution isomers of dihydroxybenzene (C6H4(OH)2): the ortho isomer catechol, the meta isomer resorcinol, and the para isomer hydroquinone.











































